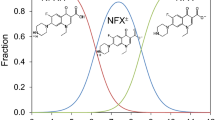
Abstract
The adsorption of antibiotic tetracycline onto silica particles was investigated. The effects of pH, electrolyte concentration and temperature on the adsorption equilibrium were examined and the data correlated with a dual site adsorption model. Adsorption of tetracycline onto approximately 9% of the adsorption sites was found to be irreversible, whereas the rest exhibited Langmuir behaviour. Increasing pH of the solution had a strong negative effect on the adsorbed amounts. Adsorption decreased also with increasing ionic strength and temperature. The enthalpy and entropy of adsorption were found to be approximately −16 and −25 J/mol, respectively.



Similar content being viewed by others

References
Adamson AW (1976) Physical chemistry of the surfaces. Wiley, New York
Atkin R, Craig VSJ, Wanless EJ, Biggs S (2003) Mechanism of cationic surfactant adsorption at the solid-aqueous interface. Adv Colloid Interface Sci 103:219–304
Chaubal MV, Payne GF, Reynolds CH, Albright RL (1995) Equilibria for the adsorption of antibiotics onto neutral polymeric sorbents: experimental and modeling studies. Biotech Bioeng 47:215–226
Figueroa RA, Leonard A, Mackay AA (2004) Modeling tetracycline antibiotic sorption to clays. Environ Sci Technol 38:476–483
Figueroa RA, Mackay AA (2005), Sorption of oxytetracycline to iron oxides and oxide-rich soils, Environ. Sci Technol 39:6664–6671
Halling-Søresen B, Nielsen SN, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998) Occurrence, fate and effect of farmaceutical substances in the environment—a review. Chemosphere 36:357–393
Lorphensri O, Intravijit J, Sabatini DA, Kibbey TCG, Osathaphan K, Saiwan C (2006) Sorption of acetaminophen, 17α-ethynyl estradiol, nalidixic acid, and norfloxacin to silica, alumina, and a hydrophobic medium. Water Res 40:1481–1491
Rabølle M, Spliid NH (2000) Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 40:715–722
Sithole BB, Guy RD (1987) Models for tetracycline in aquatic environments. Water Air Soil Pollut 32:303–321
Slishik NF, Nosach LV, Voronina OE (2004). Adsorption of tetracycline-type antibiotics on the surface of finely divided silica, Khimiya, Fizika ta. Tekhnologiya Poverkhni 10:170–174
Thiele-Brühn S (2003) Pharmaceutical antibiotic compounds in soil - a review. J Plant Nutr Soil Sci 166:145–167
Acknowledgment
Irina Turku gratefully acknowledges a personal grant from the Research Foundation of Lappeenranta University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turku, I., Sainio, T. & Paatero, E. Thermodynamics of tetracycline adsorption on silica. Environ Chem Lett 5, 225–228 (2007). https://doi.org/10.1007/s10311-007-0106-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-007-0106-1