Abstract
As candidates for tissue-independent phases of cortical and trabecular bone we consider (i) hydroxyapatite, (ii) collagen, (iii) ultrastructural water and non-collagenous organic matter, and (iv) marrow (water) filling the Haversian canals and the intertrabecular space. From experiments reported in the literature, we assign stiffness properties to these phases (experimental set I).
On the basis of these phase definitions, we develop, within the framework of continuum micromechanics, a two-step homogenization procedure: (i) at a length scale of 100–200 nm, hydroxyapatite (HA) crystals build up a crystal foam (“polycrystal”), and water and non-collagenous organic matter fill the intercrystalline space (homogenization step I); (ii) at the ultrastructural scale of mineralized tissues (5–10 microns), collagen assemblies composed of collagen molecules are embedded into the crystal foam, acting mechanically as cylindrical templates. At an enlarged material scale of 5–10 mm, the second homogenization step also accommodates the micropore space as cylindrical pore inclusions (Haversian and Volkmann canals, inter-trabecular space) that are suitable for both trabecular and cortical bone. The inputs for this micromechanical model are the tissue-specific volume fractions of HA, collagen, and of the micropore space. The outputs are the tissue-specific ultrastructural and microstructural (=macroscopic=apparent) elasticity tensors.
A second independent experimental set (composition data and experimental stiffness values) is employed to validate the proposed model. We report a small mean prediction error for the macroscopic stiffness values. The validation suggests that hydroxyapatite, collagen, and water are tissue-independent phases, which define, through their mechanical interaction, the elasticity of all bones, whether cortical or trabecular.







Similar content being viewed by others
References
Akiva U, Wagner H, Weiner S (1998) Modelling the three-dimensional elastic constants of parallel-fibred and lamellar bone. J Mater Sci 33:1497–1509
Arnoux P, Bonnoit J, Chabrand P, Jean M, Pithioux M (2002) Numerical damage models using a structural approach: application to bones and ligaments. Eur Phys J–Appl Phys 17:65–73
Ascenzi M-G (1999) A first estimation of prestress in so-called circularly fibered osteonic lamellae. J Biomech 32:935–942
Ashman R, Rho J (1988) Elastic modulus of trabecular bone material. J Biomech 21(3):177–181
Ashman R, Corin J, Turner C (1987) Elastic properties of cancellous bone: measurement by an ultrasonic technique. J Biomech 20(10):979–986
Benezra Rosen V, Hobbs L, Spector M (2002) The ultrastructure of anorganic bovine bone and selected synthetic hydroxyapatites used as bone graft substitute material. Biomaterials 23:921–928
Biltz R, Pellegrino E (1969) The chemical anatomy of bone. J Bone Joint Surg 51-A(3):456–466
Broe K, Hannan M, Kiely D, Cali C, Cupples L, Kiel D (2000) Predicting fractures using bone mineral density: a prospective study of long-term care residents. Osteoporosis Int 11:765–771
Bryant J (1983) The effect of impact on the marrow pressure of long bones in vitro. J Biomech 16(8):659–665
Bryant J (1988) On the mechanical function of marrow in long bones. Eng Med 17(2):55–58
Buckwalter J, Glimcher M, Cooper R, Recker R (1995a) Bone biology, Part I: Structure, blood supply, cells, matrix, and mineralization. The J Bone Joint Surg 77-A(8):1256–1275
Buckwalter J, Glimcher M, Cooper R, Recker R (1995b) Bone biology, Part II: Formation, form, modeling, remodeling, and regulation of cell functions. J Bone Joint Surg 77-A(8):1276–1288
Byers S, Moore A, Byard R, Fazzalari N (2000) Quantitative histomorphometric analysis of the human growth plate from birth to adulescence. Bone 27(4):495–501
Cheal E, Hipp J, Hayes W (1993) Evaluation of Finite Element analysis for prediction of the strength reduction due to metastatic lesions in the femoral neck. J Biomech 26(3):251–264
Cody D, Gorss G, Hou F, Spencer H, Goldstein S, Fyhrie D (1999) Femoral strength is better predicted by finite element models than QCT and DXA. J Biomech 32:1013–1020
Cowin S (1985) The relationship between the elasticity tensor and the fabric tensor. Mech Mater 4:137–147
Cowin S (1993) Bone stress adaptation models. J Biomech 115:528–533
Cowin S (1999) Bone poroelasticity. J Biomech 32:217–238
Cox H (1952) The elasticity and strength of paper and other fibrous materials. Brit J Appl Phys 3:72–79
Crolet J, Aoubiza B, Meunier A (1993) Compact bone: numerical simulation of mechanical characteristics. J Biomech 26(6):677–687
Currey J (1969) The relationship between the stiffness and the mineral content of bone. J Biomech 2:477–480
Currey J (1984) The mechanical adaptations of bones. Princeton University Press, Princeton, NJ, USA
Cusack S, Miller A (1979) Determination of the elastic constants of collagen by Brillouin light scattering. J Mol Biol 135:39–51
Dalstra M, Huiskes R, Erning L (1995) Development and validation of a three-dimensional finite element model of the pelvic bone. J Biomech Eng–T ASME 117:272–278
Ding M, Hvid I (2000) Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone 26:291–295
Eshelby J (1957) The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc R Soc Lond Ser A 241:376–396
Fan Z, Swadener J, Rho J, Roy M, Pharr G (2002) Anisotropic properties of human tibial cortical bone as measured by nanoindentation. J Orthopaed Res 20:806–810
Farquharson C, Houston B (1998) Growth-plate chondrocytes in normal and abnormal growth of bone. In: 1997/98 Annual Report. Roslin Institute, Edinburgh, Scotland, UK. Published online at http://www.roslin.ac.uk (cited 25 February 2004)
Fedorov F (1968) Theory of elastic waves in crystals. Plenum, New York
Fenech C, Keaveny T (1999) A cellular solid criterion for predicting the axial-shear failure properties of bovine trabecular bone. J Biomech Eng–T ASME 121:414–422
Fratzl P, Fratzl-Zelman N, Klaushofer K, Vogl G, Koller K (1991) Nucleation and growth of mineral crystals in bone studied by small-angle X-ray scattering. Calcified Tissue Int 48:407–413
Fratzl P, Schreiber S, Klaushofer K (1996) Bone mineralization as studied by small-angle X-ray scattering. Connect Tissue Res 34(4):247–254
Fung Y (2002) Celebrating the inauguration of the journal: Biomechanics and Modeling in Mechanobiology. Biomech Model Mechanobiol 1:3–4
Fyhrie D, Schaffler M (1995) The adaptation of bone apparent density to applied bone. J Biomech 28(2):135–146
Gibson L (1985) The mechanical behavior of cancellous bone. J Biomech 18:317–28
Gilmore R, Katz J (1982) Elastic properties of apatites. J Mater Sci 17:1131–1141
Gong J, Arnold J, Cohn SH (1964) Composition of trabecular and cortical bone. Anat Rec 149:325–332
Goulet R, Goldstein S, Ciarelli M, Kuhn J, Brown M, Feldkamp L (1994) The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech 27:375–389
Hellmich C, Ulm F-J (2002a) Are mineralized tissues open crystal foams reinforced by crosslinked collagen? –some energy arguments. J Biomech 35:1199–1212
Hellmich C, Ulm F-J (2002b) A micromechanical model for the ultrastructural stiffness of mineralized tissues. J Eng Mech–ASCE 128(8):898–908
Hengsberger S, Kulik A, Zysset P (2002) Nanoindentation discriminates the elastic properties of individual human bone lamellae under dry and physiological conditions. Bone 30:178–184
Hershey A (1954) The elasticity of an isotropic aggregate of anisotropic cubic crystals. J Appl Mech–T ASME 21:236–240
Hoehn L, Niven I (1985) Averages on the move. Math Mag 58:151–156
Hoffler C, Moore K, Kozloff K, Zysset P, Brown M, Goldstein S (2000) Heterogeneity of bone lamellar-level elastic moduli. Bone 26:603–609
Hollister S, Fyhrie D, Jepsen K, Goldstein S (1991) Application of homogenization theory to the study of trabecular bone mechanics. J Biomech 24:825–839
Hollister S, Brennan J, Kikuchi N (1994) A homogenization sampling procedure for calculating trabecular bone effective stiffness and tissue level stress. J Biomech 27(4):433–444
Hou F, Lang S, Hoshaw S, Reimann D, Fyhrie D (1998) Human vertebral body apparent and hard tissue stiffness. J Biomech 31:1009–1015
Huiskes R, Hollister S (1993) From structure to process, from organ to cell: recent developments of FE-analysis in orthopaedic biomechanics. J Biomech Eng–T ASME 115:520–527
Jäger I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79:1737–1746
Katz J (1980) Anisotropy of Young’s modulus of bone. Nature 283:106–107
Katz J (1981) Composite material models for cortical bone. American Society of Mechanical Engineers (ASME), New York, pp 171–184
Katz J, Ukraincik K (1971) On the anisotropic elastic properties of hydroxyapatite. J Biomech 4:221–227
Keaveny T, Wachtel E, Ford C, Hayes W (1994) Differences between the tensile and compressive strengths of bovine tibial trabecular bone depend on modulus. J Biomech 27(9):1137–1146
Kim H, Al-Hassani, S (2002) A morphological model of vertebral trabecular bone. J Biomech 35:1101–1114
Kolsky H (1953) Stress waves in solids. Clarendon, Oxford, UK
Kröner E (1971) Statistical continuum mechanics. Springer, Berlin Heidelberg New York
Lees S (1987) Considerations regarding the structure of the mammalian mineralized osteoid from viewpoint of the generalized packing model. Connect Tissue Res 16:281–303
Lees S, Klopholz D (1992) Sonic velocity and attenuation in wet cow femur for the frequency range 5 to 100 MHz. Ultrasound Med Biol 18(3):303–308
Lees S, Heeley J, Cleary P (1979). A study of some properties of a sample of bovine cortical bone using ultrasound. Calcified Tissue International 29:107–117
Lees S, Heeley J, Cleary P (1981) Some properties of the organic matrix of a bovine cortical sample in various media. Calcified Tissue Int 33:81–86
Lees S, Tao N-J, Lindsay M (1990) Studies of compact hard tissues and collagen by means of Brillouin light scattering. Connect Tissue Res 24:187–205
Lees S, Prostak K, Ingle V, Kjoller K (1994) The loci of mineral in turkey leg tendon as seen by atomic force microscope and electron microscopy. Calcified Tissue Int 55:180–189
Lotz J, Cheal E, Hayes W (1991) Fracture prediction for the proximall femur using finite element models: Part I – linear analysis. J Biomech Eng–T ASME 113:353–360
Mammone J, Hudson S (1993) Micromechanics of bone strength and failure. J Biomech 26:439–446
Martin R, Burr D, Sharkey N (2001). Skeletal tissue mechanics. Springer, Berlin Heidelberg New York
Matsushima N, Akiyama M, Terayama Y (1982) Quantitative analysis of the orientation of mineral in bone from small-angle X-ray scattering patterns. Jpn J Appl Phys 21:186–189
Mayr E (1997) This is biology –the science of the living world, 1st edn. Harvard University Press, Cambridge, MA
McCarthy R, Jeffcott L, McCartney R (1990) Ultrasound speed in equine cortical bone: effects of orientation, density, porosity and temperature. J Biomech 23(11):1139–1143
Miller A (1984) Collagen: the organic matrix of bone. Philos Tr R Soc S–B 304:455–477
Moore T, Gibson L (2001) Modeling modulus reduction in bovine bone damaged in compression. J Biomech Eng–T ASME 123:613–622
Mori T, Tanaka K (1973) Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall Mater 21(5):571–574
Niebur G, Yuen J, Hsia A, Keaveny T (1999) Convergence behavior of high-resolution finite element models of trabecular bone. J Biomech Eng–T ASME 121:629–635
Odgaard A, Kabel J, van Rietbergen B, Dalstra M, Huiskes R (1997) Fabric and elastic principal directions of cancellous bone are closely related. J Biomech 30(5):487–495
Odgaard A, Kabel J, van Rietbergen B, Huiskes R (1999) Architectural 3-D parameters and anisotropic elastic properties of cancellous bone. In: Pederson P, Bendsoe M (eds) IUTAM symposium on synthesis in bio solid mechanics. Kluwer, The Netherlands, pp33–42
Parfitt A (1984) The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in cell biology of bone. Calcified Tissue Int 36:S37–S45
Petrtyl M, Hert J, Fiala P (1996) Spatial organization of the haversian bone in man. J Biomech 29(2):161–169
Pidaparti R, Burr D (1992) Collagen fibre orientation and geometry effects on the mechanical properties of secondary osteons. J Biomech 25:869–880
Pidaparti R, Chandran A, Takano Y, Turner C (1996) Bone mineral lies mainly outside the collagen fibrils: Predictions of a composite model for osteonal bone. J Biomech 29(7):909–916
Pistoia W, van Rietbergen B, Laib A, Rüegsegger P (2001) High-resolution three-dimensional-pqct images can be an adequate basis for in-vivo μFE analysis of bone. J Biomech Eng–T ASME 132:176–183
Ramtani S, Zidi M (2001) A theoretical model of the effect of continuum danage on a bone adaptation model. J Biomech 34:471–479
Rho J, Ashman R, Turner C (1993) Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech 26(2):111–119
Rho J, Zioupos P, Currey J, Pharr G (2002) Microstructural elasticity and regional heterogeneity in human femoral bone of various ages examined by nano-indentation. J Biomech 35:189–198
Rho J-Y, Hobatho M, Ashman R (1995) Relations of mechanical properties to density and CT numbers in human bone. Med Eng Phys 17(5):347–355
Rho J-Y, Tsui T, Pharr G (1997) Elastic properties of human cortical bone and trabecular lamellar bone measured by nanoindentation. Biomaterials 18:1325–1330
Roschger P, Fratzl P, Eschberger J, Klaushofer K (1998) Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone 23(4):319–326
Rubin M, Jasiuk I, Taylor J, Rubin J, Ganey T, Apkarian R (2003) TEM analysis of the nanostructure of normal and osteoporotic bone. Bone 33:270–282
Salencon J (2001) Handbook of continuum mechanics: general concepts – thermoelasticity, 1st edn. Springer, Berlin, Germany
Sasaki N (1991) Orientation of mineral in bovine bone and the anisotropic mechanical properties of plexiform bone. J Biomech 24:57–61
Sietsema W (1995) Animal models of cortical porosity. Bone 17(4):297S–305S
Silva E, Ulm F-J (2001) A bio-chemo-mechanics approach to bone resorption and fracture. In: Karihaloo B (ed) Analytical and computational fracture mechanics of non-homogeneous materials – Proc IUTAM Symp (Cardiff, UK, 18–22 June 2001). Kluwer, Dordrecht, The Netherlands, pp 355–366
Silva M, Gibson L (1997) Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in the microstructure. Bone 21:191–199
Taylor W, Roland E, Ploeg H, Hertig D, Klabunde R, Warner M, Hobatho M-C, Rakotomanana L, Clift S (2002) Determination of orthotropic bone elastic constants using FEA and model analysis. J Biomech 35(6):767–773
Turner C, Cowin S, Rho J, Ashman R, Rice J (1990) The fabric dependence of the orthotropic elastic constants of cancellous bone. J Biomech 23(6):549–561
Uchiyama T, Tanizawa T, Muramatsu H, Endo N, Takahashi H, Hara T (1999) Three-dimensional microstructural analysis of human trabecular bonein relation to its mechanical properties. Bone 25(4):487–491
Ulrich D, van Rietbergen B, Laib A, Rüegsegger P (1999) Load transfer analysis of the distal radius from in-vivo high resolution CT-imaging. J Biomech 32:821–828
Urist M, DeLange R, Finerman G (1983) Bone cell differentiation and growth factors. Science 220:680–686
Vajjhala S, Kraynik A, Gibson L (2000) A cellular solid model for modulus reduction due to resorption of trabeculae in bone. J Biomech Eng–T ASME 122:511–515
van Rietbergen B, Weinans H, Huiskes R, Odgaard A (1995) A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech 28:69–81
van Rietbergen B, Müller R, Ulrich D, Rüegsegger P, Huiskes R (1999) Tissue stresses and strain in trabeculae of a canine proximal femur can be quantified from computer reconstructions. J Biomech 32:165–173
Wagner H, Weiner S (1992) On the relationship between the microstructure of bone and its mechanical stiffness. J Biomech 25(11):1311–20
Weiner S, Wagner H (1998) The material bone: structure – mechanical function relations. Annu Rev Mater Sci 28:271–298
Weiner S, Arad T, Traub W (1991) Crystal organization in rat bone lamellae. FEBS Lett 285(1):49–54
Weiner S, Arad T, Sabanay I, Traub W (1997) Rotated plywood structure of primary lamellar bone in the rat: orientation of the collagen fibril arrays. Bone 20:509–514
Whitehouse W (1974) The quantitative morphology of anisotropic trabecular bone. J Microsc 101:153–168
Zaoui A (1997) Structural morphology and constitutive behavior of microheterogeneous materials. In: Suquet P (ed) Continuum micromechanics. Springer, Wien New York, pp 291–347
Zaoui A (2002) Continuum micromechanics: survey. J Eng Mech–ASCE 128(8):808–816
Zysset P, Guo X, Hoffler E, Moore K, Goldstein S (1999) Elastic modulus and hardness of cortical and trabecular bone measured by nanoindentation in the human femur. J Biomech 32:1005–1012
Acknowledgements
The authors gratefully acknowledge the financial support of this study from the Max Kade Foundation, New York, NY, mediated through the Austrian Academy of Sciences (ÖAW), Vienna, Austria, which enabled the sabbatical leave of the first author at the Massachusetts Institute of Technology, Cambridge, USA, where essential parts of the research presented here were accomplished. They are also indebted to Eric Lemarchand and Emilio Silva for helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Appendix: Continuum micromechanics relationships for the definition of the two-step homogenization procedure of Figure 1
Appendix: Continuum micromechanics relationships for the definition of the two-step homogenization procedure of Figure 1
Homogenization step I
As for the polycrystal, RVE \(\hat{V}_{{\text{p}}} \), the average local strains in the two phases, ε HA and ε uw, are related to the homogeneous strains E p imposed at the boundary of \(\hat{V}_{{\text{p}}} \), by the average of the localization tensor over \(\hat{V}_{{{\text{HA}}}} \) and \(\hat{V}_{{{\text{uw}}}} \), A HA and A uw,
An estimate for the homogenized stiffness tensor is given by the classical relation of micromechanics (Zaoui 1997),
where r∈[HA,uw] is the phase index, \({\left\langle {{\left( . \right)}} \right\rangle }_{V} = 1/V{\int {_{V} {\left( . \right)}dV} }\) stands for the volume average, c r =3J k r +2K µ r denotes the (isotropic) stiffness tensor of phase r∈[HA;uw]; J, J ijkl =1/3δ ij δ kl , is the volumetric part of the fourth-order unity tensor I, I ijkl =1/2(δ ik δ jl +δ il δ kj ). δ ij is the Kronecker delta; in other words δ ij =1 for i=j, and δ ij =0 for ij. The deviatoric part of I is K=I–J. \(\hat{f}_{r} = \frac{{\hat{V}_{r} }}{{\hat{V}_{{\text{p}}} }}\) is the volume fraction of phase r, \(\hat{f}_{{{\text{HA}}}} + \hat{f}_{{{\text{uw}}}} = 1\), and \({\mathbf{A}}^{{{\text{est}}}}_{r} \) is an estimate for the localization tensor of phase r. For the polycrystal estimate, \({\mathbf{A}}^{{{\text{est}}}}_{r} \) is given by an implicit relationship,
Eshelby’s tensor S esh for spherical inclusions in a matrix with \({\mathbf{C}}^{{{\text{est}}}}_{{\text{p}}} \) reads as (p. 300 of Zaoui 1997):
with
For the solution of this implicit problem we refer to Hellmich and Ulm (2002b).
Homogenization step II
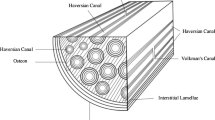
The “global” microstructural strains E m are related to the average local strains in the phases of the RVE V m (Fig. 1c) by the average concentration tensors A M, A col, and A por,
The estimate for the homogenized stiffness tensor of the microstructure is given by
where r∈[col;M;por] is the phase index, c r denotes the stiffness tensor of phase r; f r =V r /V m is the volume fraction of phase r. The matrix stiffness is known from the previous homogenization step, \({\mathbf{c}}_{{\text{M}}} = {\mathbf{C}}^{{{\text{est}}}}_{{\text{p}}} {\left( {\hat{f}_{{{\text{HA}}}} } \right)}\) (Eq. A2). Using the Mori-Tanaka (1973) scheme, the localization tensors for two families of inclusions (collagen and pores) in the HA polycrystal matrix can be estimated as
For cylindrical (collagen or micropore) inclusions in the polycrystal matrix, the non-zero components of Eshelby’s tensor \({\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{col}}}} = {\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{por}}}} = {\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{cyl}}}} \) read as
Cylindrical inclusions are to be understood in the sense of Eshelby (1957), p. 386, as inclusions of prolate spheroid type with one axis of the ellipsoid being very much longer than the other two, which are of the same length. Homogenization of the ultrastructure (Fig. 1b) is performed by analogy to Eqs. A6–A11, replacing subscript “m” by “u”, f r by \(\bar{f}_{r} \), and r∈[col,M,por] by r∈[col,M].
We have also tested the possible significance of a deviation from the cylindrical pore shape, by considering inclusions of the prolate spheroid type with length a, oriented in the x 3-direction (Fig. 1), and width b=c<a; or, in terms of a morphology parameter, ω=b/a=c/a<1. The limit case ω→0 refers to cylindrical inclusions. For prolate spheroid inclusions, the non-zero components of Eshelby’s tensor \({\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{col}}}} = {\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{por}}}} = {\mathbf{S}}^{{{\text{Esh}}}}_{{{\text{prsph}}}} \) read as (Eshelby 1957):
with an arbitrarily chosen value for a, and
where ν M=(3k M–2µ M)/(6k M+2µ M), Poisson’s ratio of the matrix.
Rights and permissions
About this article
Cite this article
Hellmich, C., Ulm, FJ. & Dormieux, L. Can the diverse elastic properties of trabecular and cortical bone be attributed to only a few tissue-independent phase properties and their interactions?. Biomech Model Mechanobiol 2, 219–238 (2004). https://doi.org/10.1007/s10237-004-0040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-004-0040-0