Abstract
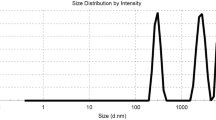
Acid hydrolysis of horn meal (obtained from raw horns of slaughtered cattle and buffaloes) yields a mixture of water soluble and low molecular weight peptides with average particle size of 3,838 nm (3.84 μm) and mean intensity of 5,243.7 nm (5.24 μm) and mean volume of 6,228.1 nm (6.23 μm). These peptides on reaction with Cr(III) yield a product that exhibits high exhaustion of chromium bath (above 92%) during tanning of hides and skins. To consolidate the results, the process was up-scaled in Central Leather Research Institute (CLRI) pilot plant for commercial trials. This material is applied directly to the pickled pelts at 8% w/w level and then the pelt is converted to wet blues and crust leathers. Control leathers were also processed concurrently using standard chrome tanning salts (8% w/w). Leathers showed the desired level of softness, fullness, shrinkage temperature and physical properties, and are comparable with control ones. Our results have indicated the use of Cr(III)–keratin complex could considerably reduce pollution load of Cr salts in leather processing.




Similar content being viewed by others
References
Backer W, Heinze H, Luck, Spahrkas H (1977) Good exhaustion of spent liquors in tanning with chrome. Das Leder 28:57
Barlett R, James J (1979) Oxidation of chromium in soils. J Environ Qual 8:31
Buljan J (1996) Pollution limits for discharge of tanning effluents into water bodies and sewers. World Leather 9:65
Chandrababu NK, Rao JR, Rao PS, Ramesh R, Babu MS, Ramasami T, Langerwerf JSA, Covington AD, Walker WP (1995) Low waste closed loop chrome tanning method. In: Proceedings of the 30th LERIG, Chennai, p 23
Chandrasekaran B (1987) An approach towards better exhaustion chrome in tanning. Leather Sci 34:91
Costas, Negulescu (1986) The recovery of basic chromium salts from the effluents resulting from the tanning operation. Ind Usoara 33:446
Covington AD, Sykes RL, Barlow JR, White ET (1983) A practical chrome recovery system using magnesium oxide. J Soc Leather Technol Chem 67:5
Dartsch PC, Kimmel R, Schmahl FW, Germann HP(1998) Nephrotoxic and hepatotoxic effects of a chromium(VI) compound in comparison to a basic chromium(III) tanning agent. World Leather 66
Davis MH, Scroggie JG (1973) Investigation of commercial chrome-tanning systems. J Soc Leather Technol Chem 57:35
Davis MH, Scrogie JG (1980) Theory and practice of direct chrome liquor recycling. Das Leder 31:1
Eary LE, Rai D (1987) Kinetics of chromium(III) oxidation to chromium(VI) by reaction with manganese dioxide. Environ Sci Technol 21:1187
France HG (1975) Recycle of tan liquor from organic acid pickle tan process. J Am Leather Chem Assoc 70:206
Hansen MB, Rydin S, Menne T, Johansen JD (2002) Quantitative aspects of contact allergy to chromium and exposure to chrome-tanned leather. Contact Dermat 47(3):127
Hansen MB, Johansen JD, Menne T (2003) Chromium allergy:significance of both Cr(III) and Cr(VI). Contact Dermat 49(4):206
IUP:2 (2000) Sampling. J Soc Leather Technol Chem 84:303
IUP:6 (2000) Measurement of tensile strength and percentage elongation. J Soc Leather Technol Chem 84:317
IUP:8 (2000) Measurement of tear load––double edge tear. J Soc Leather Technol Chem 84:327
Katz SA (1991) The analytical biochemistry of chromium. Environ Health Perspect 92:13
Kjeldahl JA (1883) A new method for determination of nitrogen in organic matter. Anal Chem 22:366
Knapp F (1921) Nature and essential character of the tanning process and of leather. J Am Leather Chem Assoc 16:658
Legesse W, Thanikaivelan P, Rao JR (2002) Underlying principles in chrome tanning: part1. Conceptual design of pickle-less tanning. J Am Leather Chem Assoc 97:475
Leonard A, Lauweys RR (1980) Carcinogenicity and mutagenicity of chromium. Mutat Res 76:227
Luck W (1980) Chrome tanning processes with particularly good exhaustion. J Am Leather Chem Assoc 75:378
Margerkurth VB (1977) Mode of action of masking agents during chrome tanning with greater exhaustion. Leder 28:155
Society of Leather Technol Chem (SLTC) (1965) Official methods of analysis. Herts
Otto G (1966) Importance of active substances with active charges retanning upper leathers. Leder 17:285
Rajamani S, Ramasami T, Langerwerf JSA, Schappman JE (1995) Environmental management in tanneries, feasible chromium recovery and reuse system. In: The 3rd international conference on appropriate waste management technologies for developing countries, NEERI, Nagpur, p 965
Rajaram R, Nair BU, Ramasami T (1995) Chromium(III) induced abnormalities in human lymphocyte cell proliferation: evidence for apoptosis. Biochem Biophys Res Commun 210:434
Ramamurthy G, Sehgal PK, Krishnan S, Kumar M (1987) Use of keratin hydrolysate for better chrome exhaustion. Leather Sci 34:224
Ramamurthy G, Sehgal PK, Kumar M (1989) Improved uptake of basic chromium salts in tanning operations using keratin hydrolysate. J Soc Leather Technol Chem 73:168
Ramasami T (2001) Approach towards a unified theory for tanning: Wilson’s dream. J Am Leather Chem Assoc 96:290
Sastry TP, Sehgal PK, Gupta KB, Kumar M (1986) Solubilised keratins as a filler in the retanning of upper leathers. Leather Sci 33:345
Sehgal PK, Sastry TP, Kumar M (1986) Studies on solubilised keratins from poultry feathers. Leather Sci 33:333
Sehgal PK, Sastry TP, Kumar M (1987) Effect of keratin filler in retanning of nappa garment leathers. Leather Sci 34:1
Sharma DC, Sharma CP, Tripathi RD (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATTC 33456. Appl Environ Microbiol 59:3771
Slabbert (1980) Recycling in the tanning industry. J Soc Leather Technol Chem 67:89
Suarez J (1981) Magnesium oxide as a neutralizing agent for chrome tanning. J Am Leather Chem Assoc 76:15
Subbrama NA (1995) Kotharis desk book on leather. In: Sadulla S (ed) Kothari Publications, Chennai, p 35
Thanikaivelan P, Rao JR, Nair BU (2000) Development of leather processing method in narrow pH profile, part1: standardization of tanning process. J Soc Leather Technol Chem 84:276
Tsou TC, Lin RJ, Yang JL (1997) Mutational spectrum induced by chromium(III) in shuttle vectors replicated in human cells: relationship to Cr(III)-DNA interactions. Chem Res Toxicol 10:962
Venier P, Montaldi A, Majone F, Bianchi V, Levis AG (1982) Cytotoxic, mutagenic and clastogenic effects of industrial chromium compounds. Carcinogenesis 3:1331
Yamini SH, Sreeram KJ, Nair BU (2004) Aggregation of mucin by chromium(III) complexes as revealed by electrokinetic and rheological studies: influence on the tryptic and o-glycanase digestion of mucin. JBSD 21:671
Acknowledgments
The authors wish to thank Dr. Venkataratnam G. S., pilot plant incharge, Chemical Engineering Lab, CLRI and to his staff members for their necessary help during the up-scaling of horn meal hydrolysate. One of the authors, R. Karthikeyan, desires to acknowledge “Council of Scientific and Industrial Research” (CSIR) for receiving Senior Research Fellowship. The author S. Balaji gratefully acknowledges the financial support received from NMITLI. The authors want to thank Ms. R. Indumathy, pre-doctoral fellow, CLRI, for helping in particle size analysis.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Process details for making wet blue
Raw material: wet salted goat skins weighing 1–1.5 kg per skin.
Soaking: (% on raw weight)
-
The skins were soaked for 6 h with 300% water and then washed with 2 changes of fresh water.
Liming: (% on soaked weight)
-
Lime 15%
-
Sodium sulphide (2%) made into a paste with 25% water and applied on flesh side and left overnight. Next day the skins were unhaired.
Reliming:
-
Lime 10%
-
Water 250%
-
Kept in the reliming bath for 3 days. Next day they were fleshed and pelt weight was noted.
Deliming: (% on pelt weight)
-
Water 150%
-
Ammonium chloride 1.5%
-
Run for 45 min, completion of deliming was checked, drained and washed with 200% water.
Pickling:
-
Water 80%
-
Salt 8%
-
Run for 10 min
-
Formic acid 0.5%
-
Run for 20 min
-
Sulphuric acid 0.75%
-
Water 20%
-
Given in four feeds at 10 min interval, finally run for 60 min. pH of the pelts was kept at 2.8.
Chrome Tanning:
-
Pickled pelts at pH 2.8
-
50% pickle water
-
8% BCS (control lot) or 8% high exhaust chrome tanning agent (expt. lot)
-
Run for 90 min
-
50% water
-
Run for 30 min
-
1% sodium formate
-
10% water
-
Run for 30 min
-
1% sodium bicarbonate
-
Run for 4 × 10 min + 60 min, pH 3.8.
Appendix 2: Process details for making crust leather
Raw material: Shaved wet blue goat leathers at 1.0 mm thickness
Washing
-
Water 250%
-
Run for 10 minutes, drain.
Neutralization
-
Neutralizing syntan 1.0%
-
Run for 20 min
-
Sodium formate 0.5%
-
Sodium bicarbonate 0.5%
-
Given in three feeds at 10 min interval, finally run for 20 min, pH should be 5.0. Then washed twice with 200% water for10 min.
Retanning, Dyeing and Fatliquoring
-
Water100%
-
Resin syntan 3%
-
Run for 20 min
-
Acid dye 2%, 30 min
-
Synthetic fatliquor 4%
-
Run for 30 min
-
Melamine based syntan 8%
-
Naphthalene based retanning syntan 8%
-
Run for 40 min
-
Fatliquor based on sulfochlorinated hydrocarbons 4%
-
Polymeric fatliquor 3%
-
Fatliquor based on synthetic and neutral oils 4%
-
Run for 40 min.
Fixing
-
Formic acid 2%
-
Water 20%
-
Given in three feeds at 10 min intervals and finally run for 30 min.
Then the leathers were piled overnight. Next day, they were set, conditioned, again set with reversible setting machine and hooked for drying. After drying, the leathers were staked and buffed using 400 grit emery paper.
Rights and permissions
About this article
Cite this article
Karthikeyan, R., Balaji, S., Chandrababu, N.K. et al. Horn meal hydrolysate–chromium complex as a high exhaust chrome tanning agent––pilot scale studies. Clean Techn Environ Policy 10, 295–301 (2008). https://doi.org/10.1007/s10098-007-0119-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-007-0119-2