Summary.
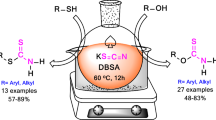
A convenient and high yielding method for the synthesis of diverse dithiocarbamates having various substituents including alkyl, aryl, heteroaryl, and alkylaryl at the thiol chain or at the amine chain or at both thiol and amine chains were developed by the one-pot reaction of mercaptans, amines, and bis(benzotriazolyl)methanethione in presence of amidine base under mild reaction conditions.
Similar content being viewed by others
References
a) Bruer H, Treuner UD (1974) US Pat 3855211; b) Rieche A, Martin D, Schade W (1963) Arch Pharm 296: 770; c) Schorr M, Duerckheimer W, Klatt P, Laemmler G, Nesemann G, Schrinner E (1969) Arzneim-Forsch 19: 1807; d) El-Shorbagi AN (2000) Arch Pharm 333: 28; e) Hanefeld W (1977) Arch Pharm 310: 409; f) Hussein MA, Shorbagi E, Khallil AR (2001) Arch Pharm 334: 305; g) Aboul-Fadl T, Hussein MA, El-Shorbagi AN, Khallil AR (2002) Arch Pharm 335: 438
a) Warshawsky A, Rogachev I, Patil Y, Baszkin A, Weiner L, Gressel J (2001) Langmuir 17: 5621; b) Farbwerke HAG (1970) Fr Pat 2015026; c) Schorr M, Duerckheimer W, Behrendt L, Duewel D (1971) Ger Pat 1 947 746; d) Nishimura K, Yasunaga T, Kanada S, Katayama S (1978) Jpn Pat 53-029932; e) Ciba-Geigy AG (1972) Br Pat 1301032; f) Henkel (1970) Fr Pat 1600071; g) Simsek R, Safak C, Erol K, Vural K (1999) Tr J Med Sc 29: 627; h) Orlinskii MM, Zimenkovskii BS (1998) J Pharma Chem 32: 516; i) Strogonova LT, Bolshakova SA, Tuzhilkova TN, Amosova SV, Ivanova NI, Tarasova OA, Alpert ML (1990) J Pharm Chem 24: 3
a) St. Georgiev V (1983) Survey of Drug Research in Immunologic Disease. In: Karger S (ed) Basel 1: 403; b) Bouzinac RM, De La Bastide RM, Charbonnier CJ, Musset M (1986) Eur Pat 179: 694; c) Gale GR (1991) Drugs Future 6: 225
a) Dhooghe M, De Kime N (2006) Tetrahedron 62: 513; b) Fernandez JMG, Mellet CO, Blanco JLJ, Mota JF, Gadelle A, Coste-Sarguet A, Defaye J (1995) Carbohy Res 268: 57
a) Mukerjee AK, Ashare R (1991) Chem Rev 91: 1; b) Boas U, Jakobsen MH (1995) J Chem Soc Chem Commun 1995; c) Elgemeie GH, Sayed SH (2001) Synthesis 1747; d) Boas U, Gertz H, Christensen JB, Heegaard PMH (2004) Tetrahedron Lett 45: 269
a) Richards LM (1947) US Pat 2423520; b) Sullivan FAV, Lindaw AC (1965) US Pat 3215703; c) Kinstler RC (1965) US Pat 3215704; d) Crich D, Quintero L (1989) Chem Rev 89: 1413; e) Barton DHR (1992) Tetrahedron 48: 2529; f) Zard SZ (1997) Angew Chem Int Ed 36: 672; g) Bongar BP, Sadavarte VS, Uppalla LS (2004) J Chem Res 9: 450; h) Greene TW, Wuts PGM (1999) Protecting Groups in Organic Synthesis, 3rd edn. Wiley Interscience, NY, p 484; i) Zhang D, Chen J, Liang Y, Zhou H (2005) Synth Commun 35: 521
a) Nishiyama Y, Tokunaga K, Kawamatsu H, Sonoda N (2002) Tetrahedron Lett 43: 1507; b) Braga AL, Martins TLC, Silveira CC, Rodrigues OED (2001) Tetrahedron 57: 3297; c) Barrett AGM, Graboski GG, Russell MA (1986) J Org Chem 51: 1012; d) Inoue T, Takanobu T, Kambe N, Ogawa A, Ryu I, Sonoda N (1994) J Org Chem 59: 5824
a) Beck G, Heitzer H (1978) US Patent 4125723; b) Barzen R, Schunack W (1980) Arch Pharm 313: 544; c) Ahlbrecht H, Kornetzky D (1998) Synthesis 775; d) Kanie K, Mizuno K, Kuroboshi M, Hiyama T (1973) Bull Chem Soc Jpn 71: 1973; e) Smith TD (1961) J Chem Soc 3164; f) Perjesi P, Sohar P (1991) Monatsh Chem 122: 1047
a) Azizi N, Aryanasab F, Torkiyan L, Ziyaei A, Saidi MR (2006) J Org Chem 71: 3634; b) Katiyar D, Tiwari VK, Tripathi RP, Srivastava AK, Chaturvedi V, Srivastava R, Srivastava BS (2003) Bio Org Med Chem 11: 4369; c) Braum JV (1902) Chem Ber 35: 3368; d) Koketsu M, Otsuka T, Ishihara H (2004) Phosph Sulf Silicon 179: 443; e) Bandgar BP, Sadavarte VS, Uppalla LS (2000) J Chem Res 9: 450
a) Katrizky AR, Singh S, Mahapatra PP, Clemense N, Kirichenko K (2005) Arkivoc 9: 63; b) Katritzky AR, Witek RM, Garcia VR, Mohapatra PP, Rogers JW, Cusido J, Abdel-Fattah AAA, Steel PJ (2005) J Org Chem 70: 7866; c) Katritzky AR, Lan X, Yang JZ, Denisko OV (1998) Chem Rev 98: 409; d) Katritzky AR, Rogovoy BV, Chassaing C, Vedensky V (2000) J Org Chem 65: 8080
a) Van der Werf S, Engberts JBFN (1970) Recl Trav Chim Pays-Bas 89: 423; b) Ulirich H, Tucker B, Sayigh AAR (1967) J Org Chem 32: 3938; c) Tiwari VK, Singh A, Mishra BB (2006) unpublished result
a) Chaturvedi D, Ray S (2006) Monatsh Chemie 137: 465; b) Chaturvedi D, Ray S (2006) Monatsh Chemie 137: 1219; c) Chaturvedi D, Ray S (2006) Monatsh Chemie 137: 311; d) Chaturvedi D, Ray S (2006) Tetrahedron Lett 47: 1307
a) For review article on DBU, see: Savoca AC, Encyclopedia for reagents for organic synthesis, edited by Paquette John Wiley & Sons, NY 2: 1497; b)Tiwari VK, Tripathi RP (2002) Ind J Chem 41B: 1681; c) Tewari N, Mishra RC, Tiwari VK, Tripathi RP (2002) Synlett 11: 1779; d) Mishra RC, Tewari N, Arora K, Ahmad R, Tripathi RP, Tiwari VK, Walter RD, Srivatava AK (2003) Comb Chem High T Scr 6: 36; e) Tewari N, Tiwari VK, Mishra RC, Tripathi RP, Srivastava AK, Ahmad R, Srivastava R, Srivastava BS (2003) Bio-Org Med Chem 11: 2911
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiwari, V., Singh, A., Hussain, H. et al. One-Pot Convenient and High Yielding Synthesis of Dithiocarbamates. Monatsh. Chem. 138, 653–658 (2007). https://doi.org/10.1007/s00706-007-0659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-007-0659-5