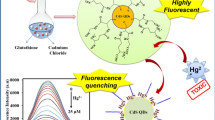
Abstract
Water-soluble and stable CdTe quantum dots (QDs) were synthesized in aqueous solution with glutathione (GSH) as the stabilizer. GSH is employed by nature to detoxify heavy metal ions. As a result of specific interaction, the fluorescence intensity of GSH-capped QDs is selectively reduced in the presence of Cr(VI). Under the optimum conditions, the relative fluorescence intensity decreases linearly with the Cr(VI) concentration in the range from 0.01 to 1.00 µg mL−1, and the detection limit is 0.008 µg mL−1. The luminescence response of the QDs to ions markedly depended on the particle size, and a new strategy for tuning the selectivity of luminescent QDs to certain ions without changing the capping layer of the QDs can be achieved by changing the crystallite size of the QDs.






Similar content being viewed by others
References
Kotas J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107:263
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Intern 31:739
For the list of drinking water contaminants in China, see: http://www.envir.gov.cn/law/standard/6.htm; for that in USA, see: http://www.epa.gov/safewater/mcl.html#mcls
Yuan D, Fu D, Wang R, Yuan J (2008) Rapid determination of chromium(VI) in electroplating waste water by use of a spectrophotometric flow injection system. Spectrochim Acta Part A 71:276
Pressman MAS, Aldstadt JH (2003) A comparative study of diffusion samplers for the determination of hexavalent chromium by sequential injection spectrophotometry. Microchem. J. 74:47
de Ruiter G, Gupta T, Van der Boom ME (2008) Selective optical recognition and quantification of parts per million levels of Cr6+ in aqueous and organic media by immobilized polypyridyl complexes on glass. J Am Chem Soc 130:2744
Xiang Y, Mei L, Li N, Tong A (2007) Sensitive and selective spectrofluorimetric determination of chromium(VI) in water by fluorescence enhancement. Anal Chim Acta 581:132
Hong S, Chen H, Wang L, Wang L (2008) Luminescent and magnetic Fe3O4/Py/PAM nanocomposites for the chromium(VI) determination. Spectrochim Acta Part A 70:449
She S, Zhou Y, Zhang L, Wang L, Wang L (2005) Preparation of fluorescent polyvinyl alcohol keto-derivatives nanoparticles and selective determination of chromium(VI). Spectrochim Acta Part A 62:711
Zhang Z, Qin W, Lu S (1995) Chemiluminescence flow system for the monitoring of chromium(VI) in water. Anal Chim Acta 318:71
Menéndez-Alonso E, Hill SJ, Foulkes ME, Crighton JS (1999) Speciation and preconcentration of CrIII and CrVI in waters by retention on ion exchange media and determination by EDXRF. J Anal At Spectrom 14:187
Séby F, Charles S, Gagean M, Garraud H, Donard OFX (2003) Chromium speciation by hyphenation of high-performance liquid chromatography to inductively coupled plasma-mass spectrometry-study of the influence of interfering ions. J Anal At Spectrom 18:1386
Anthemidis AN, Zachariadis GA, Kougoulis JS, Stratis JA (2002) Flame atomic absorption spectrometric determination of chromium(VI) by on-line preconcentration system using a PTFE packed column. Talanta 57:15
Sun Z, Liang P (2008) Determination of Cr(III) and total chromium in water samples by cloud point extraction and flame atomic absorption spectrometry. Microchim. Acta 162:121
Motomizu S, Jitmanee K, Oshima M (2003) On-line collection/concentration of trace metals for spectroscopic detection via use of small-sized thin solid phase (STSP) column resin reactors: Application to speciation of Cr(III) and Cr(VI). Anal Chim Acta 499:149
Jena BK, Raj CR (2008) Highly sensitive and selective electrochemical detection of sub-ppb level chromium(VI) using nano-sized gold particle. Talanta 76:161
Tsai MC, Chen PY (2008) Voltammetric study and electrochemical detection of hexavalent chromium at gold nanoparticle-electrodeposited indium tinoxide (ITO) electrodes in acidic media. Talanta 76:533
Arancibia V, Valderrama M, Silva K, Tapia T (2003) Determination of chromium in urine samples by complexation–supercritical fluid extraction and liquid or gas chromatography. J Chromatogr B 785:303
Han Z, Qi L, Shen G, Liu W, Chen Y (2007) Determination of chromium(VI) by surface plasmon field-enhanced resonance light scattering. Anal Chem 79:5862
Tsay JM, Pflughoefft M, Bentolila LA, Weiss S (2004) Hybrid approach to the synthesis of highly luminescent CdTe/ZnS and CdHgTe/ZnS nanocrystals. J Am Chem Soc 126:1926
Sun J, Wang LW, Buhro WE (2008) Synthesis of cadmium telluride quantum wires and the similarity of their effective band gaps to those of equidiameter cadmium telluride quantum dots. J Am Chem Soc 130:7997
Algar WR, Krull UJ (2008) Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal Bioanal Chem 391:1609
Xia YS, Zhu CQ (2009) Interaction of CdTe nanocrystals with thiol-containing amino acids at different pH: a fluorimetric study. Microchim Acta 164:29
Sun JF, Liu LH, Ren CL, Chen XG, Hu ZD (2008) A feasible method for the sensitive and selective determination of vitamin B1 with CdSe quantum dots. Microchim Acta 163:271
Dong F, Hu KW, Han HY, Liang JG (2009) A novel method for methimazole determination using CdSe quantum dots as fluorescence probes. Microchim Acta 165:195
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132
Chen HQ, Liang AN, Wang L, Liu Y, Qian BB (2009) Ultrasensitive determination of Cu2+ by synchronous fluorescence spectroscopy with functional nanoparticles. Microchim Acta 164:453
Lai SJ, Chang XJ, Fu C (2009) Cadmium sulfide quantum dots modified by chitosan as fluorescence probe for copper (II) ion determination. Microchim Acta 165:39
Fernández-Argüelles MT, Jin WJ, Costa-Fernández J, Pereir R, Sanz-Medel A (2005) Surface-modified CdSe quantum dots for the sensitive and selective determination of Cu(II) in aqueous solutions by luminescent measurements. Anal Chim Acta 549:20
Wang JH, Wang HQ, Zhang HL, Li XQ, Hua XF, Cao YC, Huang ZL, Zhao YD (2007) Purification of denatured bovine serum albumin coated CdTe quantum dots for sensitive detection of silver(I) ions. Anal Bioanal Chem 388:969
Liang JG, Ai XP, He ZK, Pang DW (2004) Functionalized CdSe quantum dots as selective silver ion chemodosimeter. Analyst 129:619
Ali EM, Zheng Y, Yu H, Ying JY (2007) Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal Chem 79:9452
Wu H, Liang J, Han H (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim. Acta 161:81
Cai ZX, Yang H, Zhang Y, Yan XP (2006) Preparation, characterization and evaluation of water-soluble l-cysteine-capped-CdS nanoparticles as fluorescence probe for detection of Hg(II) in aqueous solution. Anal Chim Acta 559:234
Li H, Zhang Y, Wang X, Xiong D, Bai Y (2007) Calixarene capped quantum dots as luminescent probes for Hg2+ ions. Mater Lett 61:1474
Banerjee S, Kar S, Santra S (2008) A simple strategy for quantum dot assisted selective detection of cadmium ions. Chem Commun 3037
Li H, Zhang Y, Wang X Q (2007) l-Carnitine capped quantum dots as luminescent probes for cadmium ions. Sensor Actuat. B-Chem.127: 593
Jin W J, Fernandez-Arguelles M T, Costa-Fernandez J M, Pereiro R, Sanz-Medel A (2005) Photoactivated luminescent CdSe quantum dots as sensitive cyanide probes in aqueous solutions. Chem Commun 883
Jin WJ, Costa-Fernández JM, Pereiro R, Sanz-Medel A (2004) Surface-modified CdSe quantum dots as luminescent probes for cyanide determination. Anal Chim Acta 522:1
Touceda-Varela A, Stevenson E I, Galve-Gasión J A, Dryden D T F, Mareque-Rivas J C (2008) Selective turn-on fluorescence detection of cyanide in water using hydrophobic CdSe quantum dots. Chem Commun 1998
Dolphin D, Avramovic O, Poulson R, Eds (1989) Glutathione. Chemical, Biochemical, and Medical Aspects, Part A. John Wiley & Sons, New York, pp 147-186
E. M. Kosower, Glutathione: Metabolism and Function. I. M. Arias, W. B. Jacoby, Eds.; Raven Press: New York, 1976; pp 1–15.
Grill E, Winnacker EL, Zenk MH (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230:674
Zhang H, Zhou Z, Yang B, Gao M (2003) The influence of carboxyl groups on the photoluminescence of mercaptocarboxylic acid-stabilized CdTe nanoparticles. J Phys Chem B 107:8
Yu WW, Quad L, Goo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 15:2854
Connett PH, Wetterhahn KE (1986) Reaction of chromium(VI) with thiols: pH dependence of chromium(VI) thio ester formation. J Am Chem Soc 108:1842
The national standards in People’s Republic of China. The determination of Cr(VI) in water-diphenycarbazine photometry, GB 1467–1487.
Henglein A (1989) Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 89:1861
Levina A, Lay PA (2004) Solution structures of chromium(VI) complexes with glutathione and model thiols. Inorg Chem 43:324
Connett PH, Wetterhahn KE (1985) In vitro reaction of the carcinogen chromate with cellular thiols and carboxylic acids. J Am Chem Soc 107:4282
Li B, Wang D, Lv J, Zhang Z (2006) Chemometrics-assisted simultaneous determination of cobalt(II) and chromium(III) with flow-injection chemiluminescence method. Spectrochim Acta Part A 65:67
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 20405009) and by the Key Project of Chinese Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Elecrtonic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 76 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Xu, C. & Li, B. Simple and sensitive detection method for chromium(VI) in water using glutathione—capped CdTe quantum dots as fluorescent probes. Microchim Acta 166, 61–68 (2009). https://doi.org/10.1007/s00604-009-0164-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0164-0