Abstract
Rheological properties of highly concentrated emulsions of the water-in-oil type were studied. Water phase (concentration approximately 91%) consists of a supersaturated aqueous solution of nitrate salts; water comprises less than 20% by mass. The average size of droplets, D, in the emulsions was varied. It was found that the emulsions are non-Newtonian liquids and flow curves measured in a sweep regime of shearing have clear low-shear-rate Newtonian domain. The complete flow curves are fitted by the Cross equation. The elastic modulus is practically constant in a very wide frequency range. Hence the viscoelastic relaxation processes might be expected at times >>100 s and in the short-term side of the curve at approximately 0.01 s. The elastic modulus (measured in oscillating testing and in elastic recovery as well) is proportional to D -2 while the Newtonian viscosity is proportional to D −1.
The time effects were observed: it was found that the emulsions behave as rheopectic materials because prolonged shearing results in an increase of viscosity in the low shear rate domain of several orders of magnitude.
Similar content being viewed by others
Introduction
Emulsions are a very popular subject of rheological studies, due to both their practical applications (as pharmaceuticals, food products, crude oil recovery, polymer blends, biological liquids and so on) and the variety of rheological effects which are observed in flow and deformations of emulsions. There are mainly two types of emulsions: oil-in-water (O/W)—a dispersion of oil droplets in a continuous water phase, and water-in-oil (W/O) type of emulsions which are a dispersion of water droplets in continuous oil phase. W/O emulsions are sometimes referred to as “invert” or “reverse” emulsions. However such unusual things as water-in-water emulsions can also exist (Capron et al. 2001).
The majority of publications, both experimental and theoretical, are devoted to emulsions with the concentration of the disperse phase less than approximately 74%, which corresponds to the limit of close packing of homogeneous spherical droplets. Models predicting rheological properties of such emulsions were proposed by Princen (1983, 1985), Princen and Kiss (1986a, 1986b), Palierne (1990), Madiedo and Gallegos (1997) and Pal (1997, 2001). Model predictions which allow one to correlate rheological (viscoelastic) properties with the structure of emulsions (primarily polymer blends) were proposed by Bousmina (1999) and Yu et al. (2002a). The theoretical model was also developed for the non-linear domain of viscoelastic behavior in emulsions (Yu et al. 2002b).
Meanwhile the current interest to studies of rheological properties of emulsions has been focused on highly (or super) concentrated emulsions as well with content of the disperse phase exceeding this limit. Viscoelastic properties of such emulsions were measured (Pons et al. 1992a, 1992b, 1995) and treated in the frames of the simplest Maxwellian relaxation mode; the model of flow of such emulsions was also proposed (Schwartz and Princen 1987). The concentration dependence of viscoelastic properties of highly concentrated emulsions were discussed based on Princen’s theory (Ponton et al. 2001).
Also, there was a series of publications devoted to highly concentrated W/O emulsions where the continuous phase used a fluorinated oil and nonionic fluorinated surfactant was based on diethylene glycol. It was shown that such highly concentrated (up to 94%) emulsions behave in a solid-like manner (Langenfield et al. 1999). Theoretical analysis predicts the main solid-like properties of such emulsions—their elastic modulus and yield stress (Babak et al. 2001). Phase behavior and structure of similar systems were studied by Rocca and Stébé (2000).
One of the interesting problems discussed in many publications of this type is the role of droplet size. There are several clear evidences demonstrating that the rheological properties are strongly influenced by the droplet size. Meanwhile this problem is far from experimental solution and often is limited by examples only. So, in the publication similar in title to our paper (Pal 1996) the experiments are limited by emulsions of two types characterized as “fine” and “coarse”. The difference of rheological behavior of these emulsions is quite evident. However, the evidence is qualitative, not quantitative.
Starting this work we had the following aims. First, our subjects for investigation were W/O super-concentrated emulsions used as “pumpable explosive”, for which there are very limited publications devoted to emulsions of this type. Second, it was considered necessary to carry out the full set of rheological measurements, including steady flow, transient regimes of deformations and viscoelastic measurements in order to obtain the complete rheological characterization of these emulsions. Third, it seemed to be very interesting to make systematic measurements with emulsions with quantitatively defined droplet sized to determine the influence of this factor on rheology of these emulsions.
Some preliminary results of rheological measurements of these emulsions were published elsewhere (Masalova et al. 2003).
Experimental
Samples
The object for investigation is a super-concentrated emulsion of the water-in-oil (W/O) type. The concentration of the disperse phase is ≈91%. This phase is solution of inorganic salts in water. Water comprises <20% by mass of this phase. The remainder of the aqueous phase is mainly inorganic nitrate salts, based on ammonium nitrate with a minor proportion of calcium and/or sodium nitrates. At room temperature, the aqueous phase is supersaturated with the nitrates remaining in the solution state while the emulsion is stable. The crystallization temperature is close to 60 °C. The density of the aqueous phase is about 1.4–1.5 g/ml.
Trace amounts (<0.5%) of pH-buffering additives and acids are also present.
The oil phase is a solution of emulsifier in hydrocarbon oils, with a density ≈0.8–0.9 g/ml. The emulsifiers comprise ≈10–20% of the oil phase.
The emulsifier is based on organic derivatives of PIBSA, especially alkanolamine derivatives. (PIBSA is an industry acronym for poly(isobutylene) succinic anhydride, which when derivatized forms an open-chain carboxylate). The PIBSA has a molecular mass in the range 900–1300.
The hydrocarbons are straight chain and cyclic paraffins, with trace amounts of arenes (alkyl-aromatics). The hydrocarbons are liquid compounds with chain lengths mainly between ten and twenty carbon atoms.
The hydrophile/lipophile balance of the emulsifier is low (between 2–4).
Emulsions used in this work are stable (no remarkable changes in rheological properties) at least during one month of storage, though some changes in the formulation can lead to instability. The emulsions do not flocculate, aggregate or cream because of the close packing of the droplets. Meanwhile the instability can arise only from some coalescence and mainly from deposition and growth of solid nitrate crystals. The study of these processes is a separate problem not touched in this work. So, all data cited in this work are related to freshly prepared emulsion.
Initially emulsion samples were prepared at temperatures about 20 °C above the crystallization temperature of the aqueous phase and then slowly cooled down to room temperature. So, the water phase is supercooled (supersaturated and therefore metastable) at temperatures of the experiment.
Emulsion formulations close or equivalent to the samples studied in this work belong to the class of so-named “liquid explosives” and used in this quality in industry (Bampfield and Cooper 1985).
Measuring the size of dispersed particles was carried out with the Mastersizer-2000 device (Malvern Instruments Co). The procedure of measuring is based on a sample dispersion under software control and the measurement of angle dependence of the intensity of scattering of a collimated He-Ne laser beam. Particle size in the range from 0.26 to 1500 μm can be measured; this range is much wider than sized of the real samples used in this work. The sized distribution calculations are based on the rigorous Mie theory and using the standard soft ware applied to the instrument.
The regulation of droplet size was achieved in the technological process of emulsion preparation. The size depends on the conditions of mixing in a vessel: speed of rotation of a stirrer and time of stirring (deformation). Varying these factors one can obtain emulsions with different droplet size. This method was used for preparing emulsions with four different droplet sizes which were then used in this study.
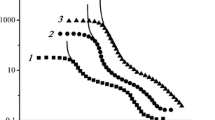
The size distribution of droplets is rather wide in all cases as shown in Fig. 1. The characteristics of samples are as follows:
Average size, μm (mean vol. weighted) | Range of sizes, μm | Maximal size, μm of 90% of droplets |
|---|---|---|
8.2 | 2.2–22 | 12.9 |
11 | 3–31 | 17.2 |
14 | 4–35 | 21.3 |
20.5 | 7–56 | 30.8 |
So, it is seen, that the droplet size for one sample varies in the limits of approximately one decimal order. Below, the size of droplets will be designated by mean volume weighted values.
Instrumentation
All rheological studies were made with a rotational stress rheometer MCR 300 (Paar Physica). The geometry of the measuring unit was “bob-in-cup” (coaxial cylinders with cone bottom of the inner one).
Smooth as well as sand-blasted surfaces of the working pair were used.
The experiments were made in the following regimes of deformation:
-
Scanning (sweep) shear rate measurements with increasing and decreasing rates in order to obtain flow curves of the emulsions in the range from 10−3 to 102 s−1
-
Creep measurements starting from the lowest stress (1 Pa) and including measurements of elastic recovery (recoil) which gave us the values of equilibrium shear modulus, Ge, at different stresses
-
Sweep oscillating regimes of deformations in the range of frequencies from 0.01 to 100 Hz; also, the amplitude of deformations was changed from approximately 1×10−4 to 10 units.
Experiments were carries on mainly at 30 °C, but some of them were repeated in the range 30–60 °C.
Results and discussion
Viscoelastic and elastic properties
Results of measuring frequency dependence of dynamic modulus is presented in Fig. 2 for the domain of low deformations (linear viscoelastic behavior). The following conclusions directly follows from these data.
Frequency dependence of dynamic modulus is rather weak and loss modulus is much lower that storage modulus. This result corresponds to many earlier published results (see, e.g., Langenfield et al. 1999) which spoke about solid-like behavior of super-concentrated emulsions. Meanwhile the existence of the plateau on the G′(ω) dependence contradicts many theoretical models predicting monotonous growth of G′ with increasing frequency (Palierne 1990; Bousmina 1999).
According to these results, low-frequency (long-time) relaxation might exist at a time scale much longer than 102 s (because the minimal experimental frequency was 0.01 Hz). However a slight increase of modulus at high frequencies might be the manifestation of the short-term relaxation process with a characteristic time lying in the range 0.02–0.08 s. It might be related to deformations inside liquid droplets or, more probable, to viscoelastic behavior of surface layers.
The role of droplet size is quite evident from Fig. 1 and will be discussed qualitatively below.
Figure 3 demonstrates that linearity of viscoelastic behavior of emulsions continues to be in a rather wide range of stress amplitudes, at least about 2 decimal orders. The same is true if one is to consider amplitude of strains but not stress as an argument of these dependencies. This is characteristic of fresh emulsions.
Figure 4 presents the results of measurements of equilibrium elastic modulus (as measured in creep and recovery experiments) in two stress domains. It is seen that fresh emulsions are linear viscoelastic materials up to rather high amplitudes of stress, no less than 100 Pa and the stress dependence of elastic modulus is not strong even at much higher stresses, though non-linearity becomes more expressed for larger droplets.
In order to clear up the role of the droplet size, Fig. 5 presents experimental data for dynamic modulus (averaged for the plateau in Fig. 1) and equilibrium modulus as a function of D−1 and D−2. It is clearly seen that the expected dependence of elastic modulus on reciprocal diameter, as proposed in some theoretical models (Princen and Kiss 1986a; Babak et al. 2001; Pal 2002) and observed by several authors for other emulsions (see, e.g., Aronson and Petko 1993; Pons et al. 1992b; Langenfield et al. 1999), does not correspond to our experimental data, whereas the dependence of elastic modulus on D−2 is really linear, i.e., elasticity is determined by the surface of droplets. Hence it is useless to try to apply these experimental results in attempts to calculate model parameters because the observed type of the G (D) dependence is different than is predicted. The reasons for the discrepancy of the experimental results may be related to the type of the emulsion used (cited experimental data were related to not so high concentrated emulsions as in our case) as well to difference in the averaging procedure for polydisperse samples.
Experimental data for elastic modulus can be successfully approximated by the empirical equation
where the numerical value of the coefficient is approximately 105 (Pa*μm2).
Viscosity
Flow curves of the emulsions with different droplet size are shown in Fig. 6. These data were obtained in the sweep regime of measuring. It is evident that the emulsions are typical non-Newtonian liquids and the low-shear range domain of initial Newtonian flow with viscosity, η 0 , definitely exists. The analogous curves were obtained at different temperatures and the master curve based on these data can be easily built in using η 0 as a fitting factor (Vinogradov and Malkin 1964) as is shown in Fig. 7.
When the flow curves are reduced into the single master curve with η 0 as a normalizing parameter, it seems that flow properties of the emulsions are similar to viscous properties of polymer melts. However the Cox-Merz rule having universal applicability to polymer melts does not work for the emulsions. Instead, complex dynamic viscosity
for the emulsions can be estimated based on the following arguments: G″ <<G′ and therefore
As seen from Fig. 2, G′≈const and then η* is proportional to ω−1 while the apparent viscosity vs shear rate dependence is quite different, as seen from Fig. 8.
Flow curves of fresh emulsions are quite satisfactory fitted by the Cross equation (Cross 1965):
where λ is some characteristic time, and the exponent n in all cases is close to 0.8. So, flow curves of the fresh emulsions look quite similar to non-Newtonian behavior of any pseudoplastic liquid. These experimental data give no hint to the existence of yielding behavior or something like this. As was shown (Masalova et al. 2003) such fitting of the data on viscous properties of the emulsions gives sufficient ground for calculating flow characteristics in pipe design.
The role of the droplet size on viscous properties is illustrated by Fig. 9 where the dependence of initial Newtonian viscosity on reciprocal diameter is shown. It is seen that—in contrast to the G(D) dependence—the η0(D) dependence is linearized if the argument is chosen as D−1. Then this dependence can be presented as
where the numerical value of the coefficient is approximately 8×104 (Pa*s*μm).
It is possible to introduce a characteristic relaxation time, θ, defined as θ=η/G. From Eqs. (1) and (3), it is seen that this characteristic time is proportional to the diameter of droplets:
and its value (in s) is of the order of the droplet size expressed in μm (because kη/kG≈1 s/μm), i.e., θ is of the order of tens of seconds. It roughly corresponds to the values of characteristic times λ in Eq. (2), i.e., this process is responsible for the non-Newtonian effect in viscous flow of the emulsions. Meanwhile there is no characteristic change in this time range on the viscoelastic curves. Then this time might be treated as not related to viscoelastic processes but to some effects connected with structural rearrangement, for example with the deformation of droplet as a whole. It explains the increase of this relaxation time with the growth of the droplet size.
Time effects
Figure 6 seems to present quite a clear image of viscous properties of the emulsion. Meanwhile the real situation appears more complicated. Data presented in Fig. 6 were obtained in the shear-rate sweep experiment in the increasing mode of shear rates sweep. What happens if we continue this measurements in the decreasing mode of shear rate sweep is shown in Fig. 10. It is seen that the results of measurements obtained in both modes of deformation coincide in the non-Newtonian domain of shearing but great increase of apparent viscosity continues down to very low shear rates in the low shear rate domain (in the Newtonian branch of the flow curve).
Then viscosity evolution was measured during prolonged application of constant low shear rate interrupted by the rather short high shear rate deformation as shown in Fig. 11. It is evident that rather slow growth of apparent viscosity takes place and this process is typical for low shear rates only, because application of high shear rate does not influence the rate of this process.
At last let us compare the results of viscosity measurements in the shear rate sweep experiment and as a limit of creep at different shear stresses. The comparison is presented in Fig. 12. It is seen that equilibrium values of viscosity can be reached only as a final of the prolonged shearing at low shear rates (or stresses). It is interesting to mention that Newtonian viscosity found from the creep data is the same and close to 107 Pa s for all droplet sizes. It reminds of the existence of the analogous very high limiting (Newtonian) viscosity value for filled liquids regardless of the viscosity of the continuous phase (Vinogradov et al. 1972). The viscosity of the bulk aqueous phase is quite low provided the temperature is sufficiently high to prevent crystal growth. The aqueous phase pours very easily and probably is no more viscous than glycerine. The viscosity of liquids increases with cooling, but at room temperature one expects the aqueous phase droplets to have low viscosities.
Very slow increase in viscosity at low shear rates (or stresses) is the phenomenon normally treated as rheopexy. According to Reiner and Scott Blair (1967) “rheopexy is the solidification of a thixotropic system by gentle and regular movement”. This is exactly what is observed in experiments presented in Figs. 10, 11, and 12 if we understand “solidification” as a large-scale increase in viscosity see comments above and maybe delete this tempting suggestion.
Time effects are typical for the emulsions under study. In this work they are of purely rheological nature and in the frames of this work these emulsions are structurally stable. Meanwhile it is necessary to remember that these emulsions are a quasi stable system due to metastability of the supersaturated disperse phase and possible instability of a multi-phase system as a whole. Therefore these emulsions are inclined to aging, the rate depending on the delicate details of the emulsion composition and temperature.
Summary
High concentrated (with the content of a disperse phase essentially exceeding the threshold of close packing) emulsions are interesting and rather new objects for rheological studies. Such systems are used as pharmaceuticals, in oil recovery, as liquid explosives, as food products, and so on. Therefore their objective rheological characterization is of interest for application. Besides these practical applications, interesting rheological effects might be observed in the study of these materials.
The subject of this work is highly concentrated emulsions of the water-in-oil type used as liquid explosives. Water phase (concentration approximately 91%) consists of a supersaturated aqueous solution of nitrate salts; water comprises less than 20% by mass. The emulsions under study did not change their rheological properties during the period of experiments and can be treated as stable.
The average size of droplets, D, in the emulsions was varied in the range 8–21 μm and the understanding of the influence of the droplet size on rheological properties of the emulsions was one of the goals of the work.
It was found that the emulsions behave as non-Newtonian liquids and flow curves measured in a sweep regime of shearing have clear low-shear-rate Newtonian domains. The flow curves in whole are fitted by the Cross equation. This equation can be used for pipe-line design.
The elastic modulus is practically constant over a very wide frequency range and in this aspect the emulsions can be treated as solid-like materials. Viscoelastic relaxation processes might be expected at times >>100 s and in a short-term side at approximately 0.01 s.
Elastic modulus (measured in oscillating testing and in elastic recovery as well) is proportional to D −2 while Newtonian viscosity is proportional to D −1.
The following time effect was observed in deformation of the emulsions. It was found that the emulsions behave as rheopexy materials because prolonged shearing results in several-order increase of viscosity in the low shear rate domain.
References
Aronson MP, Petko MF (1993) J Colloid Interface Sci 159:134–139
Babak VG, Lengfeild A, Fa N, Stébé MJ (2001) Rheological properties of highly concentrated fluorinated water-in-oil emulsions. Prog Colloid Polym Sci 118:216–220
Bampfield HA, Cooper J (1985) Emulsion explosives. In: Encyclopedia of emulsion technology, vol 3, part 7. Marcel Dekker, New York Basel
Bousmina M (1999) Rheology of polymer blends: linear model for viscoelastic emulsions. Rheol Acta 38:73–83
Capron I, Costeux S, Djaburov M (2001) Water in water emulsions: phase separation and rheology of biopolymer solutions. Rheol Acta 40:441–456
Cross MM (1965) Rheology of non-Newtonian fluids: a new flow equation for pseudoplastic systems. J Colloid Sci 20:417
Langenfield A, Schmitt V, Stébé MJ (1999) Rheological behavior of fluorinated highly concentrated reverse emulsions with temperature. J Colloid Interface Sci 218:522–528
Madiedo M, Gallegos C (1997) Rheological characterization of oil-in-water emulsions by means of relaxation and retardation spectra. Recent Res Dev Oil Chem 1:79
Masalova I, Malkin AY, Slatter P, Wilson K (2003) The rheological characterization and pipeline flow of high concentration water-in-oil emulsions. J Non-Newton Fluid Mech 112: 101-114
Pal R (1977) Dynamics of flocculated emulsions. Chem Eng Sci 52:1177
Pal R (1996) Effect of droplet size on the rheology of emulsions. AIChE J 42:3181–3190
Pal R (2001) Novel viscosity equations for emulsions of two immiscible liquids. J Rheol. 45:509–520
Pal R (2002) Novel shear modulus equation for concentrated emulsions of two immiscible elastic liquids with interfacial tension. J Non-Newton Fluid Mech 105:21–33
Palierne JF (1990) Linear rheology of viscoelastic emulsions with interfacial tension. Rheol Acta 29:204–214
Pons R, Solans C, Stébé MJ, Erra P, Ravey JC (1992a) Stability and rheological properties of gel emulsions. Prog Colloid Polym Sci 89:110–113
Pons R, Erra P, Solans C, Ravey JC, Stébé MJ (1992b) Viscoelastic properties of gel-emulsions their relationship with structure and equilibrium properties. J Phys Chem 97:12320–12335
Pons R, Solans C, Tadros TF (1995) Rheological behavior of highly concentrated oil-in-water (o/w) emulsions. Langmuir 11:1966–1971
Ponton A, Clément P, Grossiord JL (2001) Collaboration of Princen’s theory to cosmetic concentrated water-in-oil emulsions. J Rheol 45:521–526
Princen HM (1983) Rheology of foams and highly concentrated emulsions. I. Elastic properties and yield stress of a cylindrical model system. J Colloid Interface Sci 91:160–175
Princen HM (1985) Rheology of foams and highly concentrated emulsions. II. Experimental study of the yield stress and wall effects for concentrated oil-in-water emulsions. J Colloid Interface Sci 105:150–171
Princen HM, Kiss AD (1986a) Rheology of foams and highly concentrated emulsions. III. Static shear modulus. J Colloid Interface Sci 112:427–437
Princen HM, Kiss AD (1986b) Rheology of foams and highly concentrated emulsions. IV. An experimental study of the shear viscosity and yield stress of concentrated emulsions. J Colloid Interface Sci 128:176–187
Reiner M, Scott Blair DW (1967) Rheological terminology. In: Eirich FR (ed) Rheology. Theory and applications, vol 4. Academic Press, New York London, chap 9, pp 461–488
Rocca S, Stébé M-J (2000) Mixed concentrated water-oil emulsions (fluorinated/hydrogenated): formulation, properties, and structural studies. J Phys Chem B 104:10490–10497
Schwartz LW, Princen HM (1987) A theory of extensional viscosity for flowing foams and concentrated emulsions. J Colloid Interface Sci 118(1):201–211
Vinogradov GV, Malkin AY (1964) Temperature-independent viscosity characteristics of polymer systems. J Polym Sci 2A:2357–2372
Vinogradov GV, Malkin AY, Plotnikova EP, Sabsai OY, Nikolaeva NE (1972) Viscoelastic properties of filled polymers. Int J Polym Mater 2:1–27
Yu W, Bousmina M, Grmela M, Palierne JF, Zhow C (2002a) Quantitative relationship between rheology and morphology of emulsions. J Rheol 46:1381–1399
Yu W, Bousmina M, Grmela M, Zhow C (2002b) Modeling of oscillatory shear flow of emulsions under small and large deformation fields. J Rheol 46:1401–1418
Acknowledgement
The authors would like to acknowledge gratefully African Explosives Limited, the company which provided the samples, information about their structure and permission to publish the results of studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the First Annual European Rheological Conference, Guimarães, Portugal, 11–13 September 2003
Rights and permissions
About this article
Cite this article
Malkin, A.Y., Masalova, I., Slatter, P. et al. Effect of droplet size on the rheological properties of highly-concentrated w/o emulsions. Rheol Acta 43, 584–591 (2004). https://doi.org/10.1007/s00397-003-0347-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-003-0347-2