Abstract
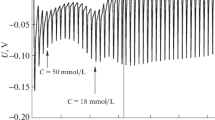
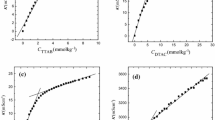
The surface tension of aqueous solutions of tetradecyl-trimethylammonium chloride (TTAC) and decyltrimethylammonium chloride (DeTAC) were measured as a function of temperature at concentrations below and above the critical micelle concentration under atmospheric pressure. The entropy and energy of adsorption from the monomeric state and from the micellar state and also the entropy and energy of micelle formation for TTAC were evaluated and compared with those of dodecyltrimethyl-ammonium chloride (DTAC). The values of ΔM W s and ΔM W u for TTAC and DTAC systems show that the micelle formation is driven by the entropy at low temperatures and by the energy at high temperatures.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 9 December 1997 Accepted: 4 March 1998
Rights and permissions
About this article
Cite this article
Hayami, Y., Ichikawa, H., Someya, A. et al. Thermodynamic study on the adsorption and micelle formation of long chain alkyltrimethylammonium chlorides. Colloid Polym Sci 276, 595–600 (1998). https://doi.org/10.1007/s003960050286
Issue Date:
DOI: https://doi.org/10.1007/s003960050286