Abstract
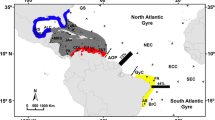
We aimed to identify biotic and abiotic factors underlying genetic structure and diversity of reef fish around Cuba. For three species, Stegastes partitus, Haemulon flavolineatum and Acanthurus tractus, we investigated the effects of shared environmental factors, such as the geography of the Cuban Archipelago, and specific characteristics, such as life history traits, on genetic structure and diversity. Samples were collected at five locations around Cuba. For S. partitus and H. flavolineatum, mitochondrial DNA and microsatellite loci were examined, whereas only mitochondrial DNA polymorphism was analyzed for A. tractus. All three species showed high genetic diversity. Mismatch distribution analyses suggest past population expansion in all species, but at different times in each species. Haplotype network and population genetic analyses suggest that: (1) S. partitus went through a recent population bottleneck in the late Pleistocene, (2) H. flavolineatum went through a population bottleneck but earlier, in the mid-Pleistocene, and (3) A. tractus has had a large and stable population size with coalescence times that go back to the late Pliocene. Genetic polymorphism in H. flavolineatum and A. tractus is homogeneous throughout the archipelago, whereas there is significant genetic structure in S. partitus. Genetic differentiation among S. partitus populations is most likely the result of the combined effects of egg type and oceanic current patterns along the Cuban coast.



Similar content being viewed by others
References
Alvarado-Bremer JR, Baker AJ, Mejuto J (1995) Mitochondrial DNA control region sequences indicate extensive mixing of swordfish (Xiphias gladius) populations in the Atlantic Ocean. Can J Fish Aquat Sci 52:1720–1732. doi:10.1139/f95-764
Amos W, Hoffman JI, Frodsham A, Zhang L, Best S, Hill AVS (2007) Automated binning of microsatellite alleles: problems and solutions. Mol Ecol Notes 7:10–14. doi:10.1111/j.1471-8286.2006.01560.x
Arriaza L, Hernández M, Lorenzo S, Olivera J, Rodas L, Montesino D, Carrillo Y, Almeida I, Simanca J, Padrón JN (2012) Modelación numérica de corrientes marinas alrededor del occidente de Cuba. Ser Ocenol 10:11–22
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Bandelt H-J, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Baums IB, Miller MW, Hellberg ME (2005) Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol 14:1377–1390. doi:10.1111/j.1365-294X.2005.02489.x
Bellwood DR, Wainwright PC (2002) The history and biogeography of fishes on coral reefs. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 5–32
Bernardi G, Lape J (2005) Tempo and mode of speciation in the Baja California disjunct fish species Anisotremus davidsonii. Mol Ecol 14:4085–4096. doi:10.1111/j.1365-294X.2005.02729.x
Bintanja R, van de Wal RSW (2008) North American ice-sheet dynamics and the onset of 100,000-year glacial cycles. Nature 454:869–872. http://www.nature.com/nature/journal/v454/n7206/suppinfo/nature07158_S1.html
Bintanja R, van de Wal RSW, Oerlemans J (2005) Modelled atmospheric temperatures and global sea levels over the past million years. Nature 437:125–128. doi:10.1038/nature03975
Borrell Y, Espinosa G, Romo J, Vázquez E, Sánchez JA, Blanco G (2004) DNA microsatellites variability and genetic differentiation among natural populations of the Cuban white shrimp Litopenaeus schmitti. Mar Biol 144:327–333
Bowen BW, Bass AL, Muss A, Carlin J, Robertson DR (2006) Phylogeography of two Atlantic squirrelfishes (Family Holocentridae): exploring links between pelagic larval duration and population connectivity. Mar Biol 149:899–913
Bowen BW, Rocha LA, Toonen RJ, Karl SA, Laboratory T (2013) The origins of tropical marine biodiversity. TREE 28:359–366. doi:10.1016/j.tree.2013.01.018
Bradbury IR, Laurel B, Snelgrove PVR, Bentzen P, Campana SE (2008) Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc R Soc B 275:1803–1809. doi:10.1098/rspb.2008.0216
Castellanos-Gell J, Robainas-Barcia A, Casane D, Chevalier-Monteagudo P, Pina-Amargós F, García-Machado E (2012) The surgeonfish, Acanthurus bahianus, has crossed the Amazon-Orinoco outflow barrier. Mar Biol 159:1561–1565. doi:10.1007/s00227-012-1942-5
Christie MR, Johnson DW, Stallings CD, Hixon MA (2010) Self-recruitment and sweepstakes reproduction amid extensive gene flow in a coral-reef fish. Mol Ecol 19:1042–1057. doi:10.1111/j.1365-294X.2010.04524.x
Claro R (ed) (1994) Ecología de los peces marinos de Cuba. Centro de Investigaciones de Quintana Roo, CIQR, Mexico
Claro R, García-Arteaga JP, Bouchon-Navarro Y, Louis M, Bouchon C (1998) Características de la estructura de las comunidades de peces en los arrecifes de Las Antillas Menores y Cuba. Avicennia 8:69–86
Claro R, Reshetnikov YS, Alcolado PM (2001) Physical attributes of coastal Cuba. In: Claro R, Lindeman KC, Parenti LR (eds) Ecology of the marine fishes of Cuba. Smithsonian Institution Press, Washington, pp 1–20
Clements KD, Gray RD, Howard Choat J (2003) Rapid evolutionary divergences in reef fishes of the family Acanthuridae (Perciformes: Teleostei). Mol Phylogenet Evol 26:190–201. doi:10.1016/S1055-7903(02)00325-1
Colin PL, Clavijo IE (1988) Spawning activity of fishes producing pelagic eggs on a shelf edge coral reef, south-western Puerto Rico. Bull Mar Sci 43:249–279
Cowen RK, Castro LR (1994) Relation of coral reef fish larval distributions to island scale circulation around Barbados, West Indies. Bull Mar Sci 54:228–244
Cowen RK, Kamazima MML, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287:857–859. doi:10.1126/science.287.5454.857
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
D’Aloia CC, Bogdanowicz SM, Harrison RG, Buston PM (2014) Seascape continuity plays an important role in determining patterns of spatial genetic structure in a coral reef fish. Mol Ecol 23:2902–2913. doi:10.1111/mec.12782
Damerau M, Matschiner M, Salzburger W, Hanel R (2014) Population divergences despite long pelagic larval stages: lessons from crocodile icefishes (Channichthyidae). Mol Ecol 23:284–299. doi:10.1111/mec.12612
DeWoody JA, Avise JC (2000) Microsatellite variation in marine, freshwater and anadromous fishes compared with other animals. J Fish Biol 56:461–473. doi:10.1006/jfbi.1999.1210
Diaz-Ferguson E, Haney R, Wares J, Silliman B (2010) Population genetics of a trochid gastropod broadens picture of Caribbean Sea connectivity. PLoS ONE 5:e12675. doi:10.1371/journal.pone.0012675
DiBattista JD, Rocha LA, Craig MT, Feldheim KA, Bowen BW (2012) Phylogeography of two closely related indo-pacific butterflyfishes reveals divergent evolutionary histories and discordant results from mtDNA and microsatellites. J Hered 103:1–13. doi:10.1093/jhered/ess056
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Gen Resour 4:359–361. doi:10.1007/s12686-011-9548-7
Eble JA, Toonen RJ, Bowen BW (2009) Endemism and dispersal: comparative phylogeography of three surgeonfishes across the Hawaiian Archipelago. Mar Biol 156:689–698. doi:10.1007/s00227-008-1119-4
Eble JA, Rocha LA, Craig MT, Bowen BW (2011) Not all larvae stay close to home: insights into marine population connectivity with a focus on the brown surgeonfish (Acanthurus nigrofuscus). J Mar Biol. doi:10.1155/2011/518516
Espinosa G, Díaz R, Matos J, Becquer U, Romo J, Borrell Y (2003) Variación aloenzimática en poblaciones cubanas del camarón blanco Litopenaeus schmitti. Rev Invest Mar 24:11–19
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes-application to human mitochondrial-DNA restriction data. Genetics 131:479–491
Eytan RI, Hellberg ME (2010) Nuclear and mitochondrial sequence data reveal and conceal different demographic histories and population genetic processes in Caribbean reef fishes. Evolution 64:3380–3397. doi:10.1111/j.1558-5646.2010.01071.x
Foster NL et al (2012) Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol Ecol 21:1143–1157
Fu YX (1997) Statistical test of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Galarza JA, Carreras-Carbonell J, Macpherson E, Pascual M, Roques S, Turner GF, Rico C (2009) The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc Natl Acad Sci USA 106:1473–1478. doi:10.1073/pnas.0806804106
García-Cagides A, Claro R, Koshelev YBV (1994) Reproducción. In: Claro R (ed) Ecología de los peces marinos de Cuba. Centro de Investigaciones de Quintana Roo, CIQR, Mexico, pp 187–209
García-Machado E, Robainas A, Espinosa G, Páez J, Verdecia N, Monnerot M (2001) Allozyme and mitochondrial DNA variation in Cuban populations of the shrimp Farfantepenaeus notialis (Crustacea:Decapoda). Mar Biol 138:701–707
González-Sansón G, Aguilar C (2003) Variaciones espaciales y temporales en la abundancia de las especies domunantes de peces de arrecife de coral en la costa de Ciudad de La Habana, Cuba. Rev Invest Mar 24:99–110
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www.unil.ch/izea/softwares/fstat.html
Grant WS, Bowen BW (1998) Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J Hered 89:415–426
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi:10.1080/10635150390235520
Haug GH, Tiedemann R (1998) Effect of the formation of the Isthmus of Panama on Atlantic Ocean thermohaline circulation. Nature 393:673–676
Hedgecock D (1994) Does variance in reproductive success limit effective population sizes of marine organisms? In: Beaumont AR (ed) Genetic and evolution of aquatic organisms. Chapman & Hall, London, pp 122–134
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Heller R, Siegismund HR (2009) Relationship between three measures of genetic differentiation GST, DEST and G’ST: how wrong have we been? Mol Ecol 18:2080–2083. doi:10.1111/j.1365-294X.2009.04185.x
Hepburn RI, Sale PF, Dixon B, Heath DD (2009) Genetic structure of juvenile cohorts of bicolor damselfish (Stegastes partitus) along the Mesoamerican barrier reef: chaos through time. Coral Reefs 28:277–288. doi:10.1007/s00338-008-0423-2
Hofmann EE, Worley SJ (1986) An investigation of the circulation of the Gulf of Mexico. J Geophys Res 91:14221–14236
Hogan JD, Thiessen RJ, Heath DD (2010) Variability in connectivity indicated by chaotic genetic patchiness within and among populations of a marine fish. Mar Ecol Prog Ser 417:263–275. doi:10.3354/meps08793
Hogan JD, Thiessen RJ, Sale PF, Heath DD (2012) Local retention, dispersal and fluctuating connectivity among populations of a coral reef fish. Oecologia 168:61–71. doi:10.1007/s00442-011-2058-1
Horne JB, Lv H, Choat JH, Robertson DR (2008) High population connectivity across the Indo-Pacific: congruent lack of phylogeographic structure in three reef fish congeners. Mol Phylogenet Evol 49:629–638. doi:10.1016/j.ympev.2008.08.023
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332. doi:10.1111/j.1755-0998.2009.02591.x
Hudson RR (2000) A new statistic for detecting genetic differentiation. Genetics 155:2011–2014
Hudson RR, Boos DD, Kaplan NL (1992) A statistical test for detecting geographic subdivision. Mol Biol Evol 9:138–151
Klanten OS, Choat JH, Lv H (2007) Extreme genetic diversity and temporal rather than spatial partitioning in a widely distributed coral reef fish. Mar Biol 150:659–670. doi:10.1007/s00227-006-0372-7
Kuhner MK (2006) LAMARC 2.0: maximum likelihood and Bayesian estimation of population parameters. Bioinformatics 22:768–770
Lee WJ, Conroy J, Howell WH, Kocher TD (1995) Structure and evolution of teleost mitochondrial control regions. J Mol Evol 41:54–66
Leis JM (1991) The pelagic stage of reef fishes: the larval biology of coral reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press Inc., San Diego, p 725
Leis JM, Rennis DS (1983) The larvae of Indo-Pacific coral reef fishes. University of Hawaii Press, Honolulu
Lessa EP (1990) Multidimensional analysis of geographic genetic structure. Syst Zool 39:242–252
Lessios HA, Robertson DR (2006) Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc R Soc B 273:2201–2208. doi:10.1098/rspb.2006.3543
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Lindeman KC, Lee TN, Wilson WD, Claro R, Ault JS (2001) Transport of larvae originating in Southewest Cuba and the Dry Tortugas: evidence for partial retention in grunts and snappers. Proc Gulf Caribb Fish Inst 52:732–747
Mantel NA (1967) The detection of disease clustering and generalized regression approach. Cancer Res 27:209–218
McCartney MA, Keller G, Lessios HA (2000) Dispersal barriers in tropical oceans and speciation in Atlantic and eastern Pacific sea urchins of the genus Echinometra. Mol Ecol 9:1391–1400
McCusker MR, Bentzen P (2010) Positive relationships between genetic diversity and abundance in fishes. Mol Ecol 19:4852–4862. doi:10.1111/j.1365-294X.2010.04822.x
Meirmans PG (2006) Using the AMOVA framework to estimate a standardized genetic differentiation measure. Evolution 60:2399–2402
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl 13:146–158. doi:10.1890/1051-0761
Paris CB, Cowen RK, Claro R, Lindeman KC (2005) Larvae transport pathways from Cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar Ecol Prog Ser 296:93–106. doi:10.3354/meps296093
Pianka ER (1978) Evolutionary ecology. Harper and Row, New York
Pillans B, Chappell J, Naish TR (1998) A review of the Milankovitch climatic beat: template for Plio-Pleistocene sea-level changes and sequence stratigraphy. Sediment Geol 122:5–21
Planes S, Galzin R, Bonhomme F (1996) A genetic metapopulation model for reef fishes in oceanic islands: the case of the surgeonfish, Acanthurus triostegus. J Evol Biol 9:103–117. doi:10.1046/j.1420-9101.1996.9010103.x
Polzin T, Daneschmand SV (2003) On Steiner trees and minimum spanning trees in hypergraphs. Oper Res Lett 31:12–20
Portnoy DS, Hollenbeck CM, Renshaw MA, Cummings NJ, Gold JR (2013) Does mating behaviour affect connectivity in marine fishes? Comparative population genetics of two protogynous groupers (Family Serranidae). Mol Ecol 22:301–313. doi:10.1111/mec.12128
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Puebla O, Bermingham E, McMillan WO (2012) On the spatial scale of dispersal in coral reef fishes. Mol Ecol 21:5675–5688. doi:10.1111/j.1365-294X.2012.05734.x
Purcell JFH, Cowen RK, Hughes CR, Williams DA (2006) Weak genetic structure indicates strong dispersal limits: a tale of two coral reef fish. Proc R Soc B 273:1483–1490. doi:10.1098/rspb.2006.3470
Purcell JFH, Cowen RK, Hughes CR, Williams DA (2009) Population structure in a common Caribbean coral-reef fish: implications for larval dispersal and early life-history traits. J Fish Biol 74:403–417. doi:10.1111/j.1095-8649.2008.02078.x
Ramos-Onsins SE, Rozas J (2002) Statistical properties of new neutrality tests against population growth. Mol Biol Evol 19:2092–2100
Randall JE (1961) A contribution to the biology of the convict surgeonfish of the Hawaii Islands, Acanthurus triostegus sandvicensis. Pac Sci 15:215–272
Raymo ME (1994) The initiation of Northern Hemisphere glaciation. Annu Rev Earth Planet Sci 22:353–383. doi:10.1146/annurev.ea.22.050194.002033
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Riginos C, Nachman MW (2001) Population subdivision in marine environments: the contributions of biogeography, geographical distance and discontinuous habitat to genetic differentiation in a blennioid fish, Axoclinus nigricaudus. Mol Ecol 10:1439–1453. doi:10.1046/j.1365-294X.2001.01294.x
Riginos C, Douglas KE, Jin Y, Shanahan DF, Treml EA (2011) Effects of geography and life history traits on genetic differentiation in benthic marine fishes. Ecography 34:566–575
Riginos C, Buckley YM, Blomberg SP, Treml EA (2014) Dispersal capacity predicts both population genetic structure and species richness in reef fishes. Am Nat 184:52–64. doi:10.1086/676505
Robainas A, Espinosa G, Hernández D, García-Machado E (2005) Temporal variation of the population structure and genetic diversity of Farfantepenaeus notialis assessed by allozyme loci. Mol Ecol 14:2933–2942. doi:10.1111/j.1365-294X.2005.02613.x
Robertson DR (1985) Sexual-size dimorphism in surgeon fishes. In: Proceedings of the 5th International Congress on Coral Reefs, vol 5, pp 403–408
Robertson DR (1990) Differences in the seasonalities of spawning and recruitment of some small neotropical reef fishes. J Exp Mar Biol Ecol 144:49–62. doi:10.1016/0022-0981(90)90019-9
Robertson DR, Green DG, Victor BC (1988) Temporal coupling of production and recruitment of larvae of a caribbean reef fish. Ecology 69:370–381. doi:10.2307/1940435
Robertson DR, Ackerman JL, Choat JH, Posada JM, Pitt J (2005) Ocean surgeonfish Acanthurus bahianus. I. The geography of demography. Mar Ecol Prog Ser 295:229–244. doi:10.3354/meps295229
Rocha LA, Bass AL, Robertson DR, Bowen BW (2002) Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Mol Ecol 11:243–252. doi:10.1046/j.0962-1083.2001.01431.x
Rocha LA, Robertson DR, Roman J, Bowen BW (2005) Ecological speciation in tropical reef fishes. Proc R Soc B 272:573–579. doi:10.1098/2004.3005
Rocha LA, Rocha CR, Robertson DR, Bowen BW (2008) Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. BMC Evol Biol 8:157. doi:10.1186/1471-2148-8-157
Roff DA, Bentzen P (1989) The statistical analysis of mitochondrial DNA polymorphisms: X 2 and the problem of small samples. Mol Biol Evol 6:539–545
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Rousset F (2008) genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106. doi:10.1111/j.1471-8286.2007.01931.x
Salas E, Molina-Ureña H, Walter RP, Heath DD (2010) Local and regional genetic connectivity in a Caribbean coral reef fish. Mar Biol 157:437–445. doi:10.1007/s00227-009-1330-y
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning—a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Schlueter MA (1998) Population genetic structure and gene flow within three coral reef fish species in the Florida Keys. Dissertation, Miami University
Schmidt D, Pool J (2002) The effect of population history on the distribution of the Tajima’s D statistic. Cornell University Press, Ithaca, p 8
Schneider S, Excoffier L (1999) Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rate vary among sites: application to human mitochondrial DNA. Genetics 152:1079–1089
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Selkoe KA, Toonen RJ (2011) Marine connectivity: a new look at pelagic larval duration and genetic metrics of dispersal. Mar Ecol Prog Ser 436:291–305. doi:10.3354/meps09238
Severance EG, Karl SA (2006) Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Mar Biol. doi:10.1007/s00227-006-0332-2
Shulman MJ, Bermingham E (1995) Early life histories, ocean currents, and the population genetics of Caribbean reef fishes. Evolution 49:897–910
Sponaugle S, Cowen RK (1996) Larval supply and patterns of recruitment for two caribbean reef fishes, Stegastes partitus and Acanthurus bahianus. Mar Freshw Res 47:433–447
Sponaugle S, Fortuna J, Grorud K, Lee T (2003) Dynamics of larval fish assemblages over a shallow coral reef in the Florida Keys. Mar Biol 143:175–189
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Taylor MS, Hellberg ME (2003) Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science 299:107–109
Taylor MS, Hellberg ME (2006) Comparative phylogeography in a genus of coral reef fishes: biogeographic and genetic concordance in the Caribbean. Mol Ecol 15:695–707. doi:10.1111/j.1365-294X.2006.02820.x
Valdés-Muñoz E, Mochek AD (1994) Estructura etológica de las comunidades de peces. In: Claro R (ed) Ecología de los peces marinos de Cuba. Centro de Investigaciones de Quintana Roo, CIQR, Mexico, pp 143–162
Valdés-Muñoz E, Mochek AD (2001) Behavior of the marine fishes of the Cuban shelf. In: Claro R, Lindeman KC, Parenti LR (eds) Ecology of the marine fishes of Cuba. Smithsonian Institution Press, Washington, pp 58–71
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Victoria del Río I, Penié I (1998) Hidrología. In: Vales M, Alvarez A, Montes L, Avila A (eds) Estudio nacional sobre la diversidad biológica en la República de Cuba. PNUMA/CENBIO/IES/AMA/CITMA, La Habana, pp 117–125
Villegas-Hernández H, González-Salas C, Aguilar-Perera A, López-Gómez MJ (2008) Settlement dynamics of the coral reef fish Stegastes partitus, inferred from otolith shape and microstructure analysis. Aquat Biol 1:249–258. doi:10.3354/ab00026
Villegas-Sánchez CA, Rivera-Madrid R, Arias-González JE (2010) Small-scale genetic connectivity of bicolor damselfish (Stegastes partitus) recruits in Mexican Caribbean reefs. Coral Reefs 29:1023–1033. doi:10.1007/s00338-010-0643-0
Vollmer SV, Palumbi SR (2007) Restricted gene flow in the caribbean staghorn coral Acropora cervicornis: implications for the recovery of endangered reefs. J Hered 98:40–50
Wang IJ (2010) Recognizing the temporal distinctions between landscape genetics and phylogeography. Mol Ecol 19:2605–2608. doi:10.1111/j.1365-294X.2010.04715.x
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wellington GM, Victor BC (1989) Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae). Mar Biol 101:557–567
White C, Selkoe KA, Watson J, Siegel DA, Zacherl DC, Toonen RJ (2010) Ocean currents help explain population genetic structure. Proc R Soc B 277:1685–1694. doi:10.1098/rspb.2009.2214
Williams DA, Purcell J, Hughes CR, Cowen RK (2003) Polymorphic microsatellite loci for population studies of the bicolor damselfish, Stegastes partitus (Pomacentridae). Mol Ecol Notes 3:547–549
Williams DA, Purcell J, Cowen RK, Hughes CR (2004) Microsatellite multiplexes for high-throughput genotyping of French grunts (Haemulon flavolineatum, Pisces: Haemulidae) and their utility in other grunt species. Mol Ecol Notes 4:46–48
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420
Zaykin DV, Pudovkin AI (1993) Two programs to estimate significance of X 2 values using pseudo-probability test. J Hered 84:152
Zink RM, Barrowclough GF (2008) Mitochondrial DNA under siege in avian phylogeography. Mol Ecol 17:2107–2121. doi:10.1111/j.1365-294X.2008.03737.x
Acknowledgments
We would like to thank Oscar Valmaña and Luis Sánchez for essential support with field work, and Gaspar González Sansón and Consuelo Aguilar Betancourt for valuable discussions on the ecology of marine fish. We also acknowledge Roamsy Volta for assistance with figure production. We thank also the three reviewers and the editor for their valuable comments and suggestions. This study was partially financed by the Research Grant A4139-1 from the International Foundation for Science awarded to ARB and the Embassy of France in Cuba.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: O. Puebla.
Reviewed by C. A. Villegas Sanchez and undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castellanos-Gell, J., Robainas-Barcia, A., Pina-Amargós, F. et al. Genetic diversity of reef fishes around Cuba: a multispecies assessment. Mar Biol 163, 165 (2016). https://doi.org/10.1007/s00227-016-2935-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2935-6