Abstract
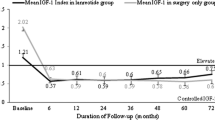
The aim of the present multicentric, open-label, non-comparative study was to evaluate the role of octreotide long-acting repeatable (LAR) as primary therapy for the treatment of GH-secreting pituitary macroadenomas. The patients received octreotide LAR 20 mg every 4 weeks for 12 weeks; afterwards the dose was confirmed or adjusted at 30 mg every 4 weeks, for the remaining 12 weeks, for responder or non-responder patients, respectively. Responder patients continued the study until 48 weeks. Twenty-one naïve active acromegalic patients were enrolled. In all patients, GH profile, IGF-I levels and magnetic resonance imaging (MRI) were evaluated at baseline and during treatment. The ability of octreotide LAR to decrease mean GH <2.5 μg/l and/or normalize IGF-I levels, adjusted for age and gender, was defined respectively as total or partial success. Total success was achieved in 5/21 (23.8%), 6/20 (30%) and 4/14 (28.6%) patients after 12, 24 and 48 weeks; partial success in 7/21 (33.3%), 9/20 (45%) and 9/14 (64%) patients at 12, 24 and 48 weeks according to GH levels, while according to IGF-I levels in 7/21 (33.3%), 7/20 (35%) and 5/14 (35.7%) patients at 12, 24 and 48 week. Tumor size was notably decreased after treatment with octreotide LAR: in 16 macroadenoma patients completing the study, the tumor sizes were 1609±1288, 818±616 (49.1±23.7%) and 688±567 mm3 (54.6±24.4%) at baseline, 24 and 48 weeks. This study shows that oc-treotide LAR is effective in suppressing GH/IGF-I secretion and inducing tumor shrinkage in GH-secreting macroadenomas in a 48-week treatment. Octreotide LAR could be used as primary therapy in patients harbouring large pituitary tumors, who are less likely to be cured by neurosurgery.
Similar content being viewed by others
References
Melmed S, Ho K, Klibanski A, Reichlin S, Thorner M. Recent advances in pathology, diagnosis and management of acromegaly. J Clin Endocrinol Metab 1995, 80: 3395–402.
Barkan AL, Halasz I, Dornfeld KJ, et al. Pituitary irradiation is ineffective in normalizing plasma Insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol 1997, 82: 3187–91.
Melmed S, Casanueva FF, Cavagnini F, et al. Acromegaly Treatment Consensus Workshop Participants. Guidelines for acromegaly management. J Clin Endocrinol Metab 2002, 87: 4054–8.
Ayuk J, Clayton RN, Holder G, Sheppard MC, Stewart PM, Bates AS. Growth Hormone and pituitary radiotherapy, but not serum Insulin-Like Growth Factor-I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab 2004, 89: 1613–7.
Nabarro JDN Acromegaly. Clin Endocrinol (Oxf) 1987, 26: 481–512.
Giustina A, Casanueva FF, Cavagnini F, et al. Diagnosis and treatment of acromegaly complications. J Endocrinol Invest 2003, 26: 1242–7.
Lim MJ, Barkan AL, Buda AJ. Rapid reduction of left ventricular hypertrophy in acromegaly after suppression of growth hormone hypersecretion. Ann Intern Med 1992, 117: 719–26.
Bates AS, Van’t Hoff W, Jones JM, Clayton RN. An audit of outcome of treatment in acromegaly. Q J Med 1993, 86: 293–9.
Ronchi CL, Orsi E, Giavoli C, et al. Evaluation of insulin resistance in acromegalic patients before and after treatment with somatostatin analogues. J Endocrinol Invest 2003, 26: 533–8.
Melmed S, Jackson I, Kleinberg D, Klibanski A. Current treatment guidelines for acromegaly. J Clin Endocrinol Metab 1998, 83: 2646–52.
Sheaves R, Jenkins P, Blackburn P, et al. Outcome of transsphenoidal surgery for acromegaly using strict criteria for surgical cure. Clin Endocrinol (Oxf) 1996, 45: 407–13.
Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf) 2003 58: 86–91.
Colao A, Pivonello R, Cappabianca P, Vitale G, Lombardi G. The treatment algorithm of acromegaly. J Endocrinol Invest 2003, 26 (Suppl 8): 39–45.
Fahlbusch R, Honegger J, Schott W, Buchfelder M Results of surgery in acromegaly. In: Wass JAH eds. Treating ac-romegaly. Bristol, UK: Journal of Endocrinology Ltd. 1994, 49–54.
Newman CB, Melmed S, Snyder PJ, et al. Safety and efficacy of long term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients — a clinical research center study. J Clin Endocrinol Metab 1995, 80: 2768–75.
Giustina A, Barbara A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000, 85: 526–9.
Attanasio R, Barausse M, Cozzi R. GH/IGF-I normalization and tumor shrinkage during long-term treatment of acromegaly by lanreotide. J Endocrinol Invest 2001, 24: 209–16.
Gillis JC, Noble S, Goa KL. Octreotide long-acting release (LAR): a review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs 1997, 53: 681–99.
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR: a promising therapeutic tool in the management of acromegalic patients. Metabolism 1996, 8 (Suppl 1): 67–71.
Newman CB, Melmed S, George A, et al. Octreotide as primary therapy in acromegaly. J Clin Endocrinol Metab 1998, 83: 3034–40.
Robbins RJ. Depot somatostatin analogs — a new first line therapy for acromegaly. J Clin Endocrinol Metab 1997, 82: 15–7.
Bevan JS, Atkin SL, Atkinson AB, et al. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab 2002, 87: 4554–63.
Ezzat S, Redelmeier DA, Gnehm M, Harris AG. A prospective multicenter octreotide dose response study in the treatment of acromegaly. J Endocrinol Invest 1995, 18: 364–9.
Freda PU, Wardlaw SL, Post KD. Long-term endocrinologic follow-up after transsphenoidal surgery for acromegaly. J Neurosurg 1998, 89: 353–8.
Swearingen B, Barker FG 2nd, Katznelson L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 1998, 83: 3419–26.
Colao A, Lombardi G Growth hormone and prolactin excess. Lancet 1998, 352: 1455–61.
Colao A, Ferone D, Marzullo P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocri-nol Metab 2001, 86: 2779–86.
Lundin P, Engstrom BE, Karlsson FA, Burman P. Long-term octreotide therapy in growth hormone-secreting pituitary adenomas: evaluation with serial MR. AJNR Am J Neuroradiol 1997, 18: 765–77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grottoli, S., Celleno, R., Gasco, V. et al. Efficacy and safety of 48 weeks of treatment with octreotide LAR in newly diagnosed acromegalic patients with macroadenomas: An open-label, multicenter, non-comparative study. J Endocrinol Invest 28, 978–983 (2005). https://doi.org/10.1007/BF03345335
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345335