Summary
3TC (GR109714X) is a new cytosine dideoxynucleoside analogue that has been shown to have in vitro activity against a variety of laboratory and clinical strains of HIV-1 including zidovudine (ZDV)-resistant isolates. Preclinical studies suggest that 3TC may have fewer adverse effects than other antiretroviral nucleoside analogues. An initial bioavailability study demonstrated that 3TC was rapidly and extensively absorbed following oral administration.
Because the bioavailability of other nucleoside analogues is significantly affected when taken with food, we conducted a randomised 2-way crossover study to determine the pharmacokinetics of 3TC following a single oral 50mg dose with and without a standard fat meal in 12 HIV-infected men.
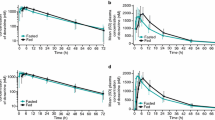
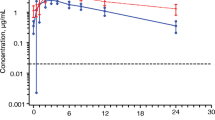
In the absence of food, 3TC is rapidly absorbed with a time taken to reach maximum serum concentration (tmax) of 0.88 ± 0.25 hours and a mean maximum serum concentration (Cmax) of 513 ± 215 ng/ml. In the presence of a standard fat meal, the tmax is delayed (3.15 ± 1.27 hours, p = 0.0001) and the Cmax is decreased (273 ± 56 ng/ml, p = 0.0017). The total amount of drug absorbed into the systemic circulation as indicated by the area under the curve over 24 hours (AUC) is not significantly different in the fed and fasted states (fasting AUC = 1677 ±424 ng ∙ h/ml, fed AUC = 1548 ± 247 ng ∙ h/ml, p = 0.1347). This demonstrates that although absorption of 3TC is delayed and the maximal concentration is reduced in the presence of a standard fat meal, the amount of drug absorbed is not altered. The intersubject variability is also limited and certainly not increased when 3TC is taken with a meal. Because the activity profile is probably dependent upon systemic activity and not absorption rate, these data suggest that 3TC may be effectively administered to patients in fasting or fed states.
Similar content being viewed by others
References
Cammack N, Rouse P, Mann CL, Reid PJ, Boehme RE, et al. Cellular metabolism of (-) enantiomeric 2′-deoxy-3′-thiacyti-dine (3TC). Biochemical Pharmacology 43: 2049–2064, 1992
Coates JAV, Cammack N, Jenkinson HJ, Mutton IM, Pearson BA, et al. The separated enantiomers of 2′-deoxy-3′-thiacyti-dine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrobial Agents and Chemotherapy 36: 202–205, 1992
Harker A, Evans GL, Morris DM, Hawley AE. A high performance liquid chromatographic assay for 3TC in human serum. Published abstract, VIII International Conference on AIDS/III STD World Congress, Amsterdam, 1992
Hart GJ, Boehme RE, Cameron JM. The effect of (-)-2′-deoxy-3′-thiacytidine (3TC) 5′-triphosphate on DNA polymerases alpha, beta and gamma. Antimicrobial Agents and Chemotherapy, in press, 1993
Lotterer E, Ruhnke M, Trautmann M, Beyer R, Bauer FE. Decreased and variable systemic availability of zidovudine in patients with AIDS if administered with a meal. European Journal of Clinical Pharmacology 40: 305–308, 1991
McDougal JS, Martin LS, Cort SP, Mozen M, Heldebrant CM, et al. Thermal inactivation of the acquired immunodeficiency syndrome virus, Human T Lymphotropic Virus-III/Lymph-adenopathy-Associated Virus, with special reference to antihemophilic factor. Journal of Clinical Investigation 76: 875–877, 1985
National Committee for Clinical Laboratory Standards (NCCLS), protection of laboratory workers from infectious disease transmitted by blood, body fluids, and tissue. NCCLS Document M29-T2, Vol. 11, No. 14, 1991
Resnick L, Veren K, Salahuddin SZ, Tondreau S, Markham PD. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. Journal of the American Medical Association 255: 1887–1891, 1986
Shyu WC, Knupp CA, Pittman KA, Dunkle L, Barbhaiya RH. Food-induced reduction in bioavailability of didanosine. Clinical Pharmacology and Therapeutics 50: 503–507, 1991
Unadkat JD, Collier AC, Crosby SS, Cunningham DC, Opheim KE, et al. Pharmacokinetics of oral zidovudine (azidothymi-dine) in patients with AIDS when administered with and without a high-fat meal. AIDS 4: 229–232, 1990
van Leeuwen R, Lange JMA, Hussey EK, Donn KH, Hall ST, et al. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection; a phase 1 study. AIDS, in press, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Angel, J.B., Hussey, E.K., Hall, S.T. et al. Pharmacokinetics of 3TC (GR109714X) Administered With and Without Food to HIV-infected Patients. Drug Invest. 6, 70–74 (1993). https://doi.org/10.1007/BF03258455
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258455