Abstract
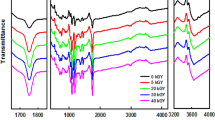
We have used FT-IR spectra to explain the effects of hydrogen bonding between chitosan and polycaprolactam (PA6). A dynamic mechanical analysis study suggested that the optimum chitosan and PA6 miscibility under the conditions of this experiment were obtained at a blending ratio of 40∶60. We studied the thermal degradation of chitosan blended with PA6 (chitosan/PA6) by thermogravimetric analysis and kinetic analysis (by the Ozawa method). Dry chitosan and PA6 exhibited a single stage of thermal degradation and chitosan/PA6 blends having > 20 wt% PA6 exhibited at least two stages of degradation. In chitosan/PA6 blends, chitosan underwent the first stage of thermal degradation; the second stage proceeded at a temperature lower than that of PA6, because the decomposition product of chitosan accelerated the degradation of PA6. The activation energies of the blends were between 130 and 165 kJ/mol, which are also lower than that of PA6.
Similar content being viewed by others
References
M. Hasegawa, A. Isogai, F. Onabe, M. Usuda, and R. Atalla,J. Appl. Polym. Sci.,45, 1873 (1992).
S. W. Shalaby,Biomedical Polymers, Carl Hanser Verlag, New York, 1994.
S. Bartnicki-Garcia and W. J. Nickerson,Biochim. Biophys. Acta.,5, 102 (1962).
S. Aiba,Makromol. Chem.,194, 65 (1993).
R. A. A. Muzzarelli,Natural Chelating Polymers, Pergamon, New York, 1973.
R. A. A. Muzzarelli and E. R. Pariser,Proceeding of First Internation Conference on Chitin/Chitosan, MIT Sea Grant ReportMITSG 78-7, May, 1978.
W. A. Bough,Food Product Development,11, 90 (1977).
T. D. Rathke and A. M. Hudson,Rev. Macromol. Chem. Phys.,C34, 375 (1994).
J. Hosokawa,Food Packag.,2, 38, (1990).
R. Muzzarelli, G. Bjagini, and C. Rizzoli,Biomaterials,10, 598 (1989).
M. Miya, S. Yoshikawa, R. Iwamoto, and S. Mima,Koubunshi Ronbunshu,40, 645 (1983).
R. J. Samuels,J. Polym. Sci.: Polym. Phys. Ed.,19, 1081 (1981).
J. A. Ratto, C. C. Chen, and R. B. Blumstein,J. Appl. Polym. Sci.,59, 1451 (1996).
J. Hosokawa, M. Nishiyama, K. Yoshihara, T. Kubo, and A. Terabe,Ind. Eng. Chem. Res.,30, 788 (1991).
I. Vieira, V. L. S. Severgnini, D. J. Mazerz, M. S. Soldi, and E. A. Pinheiro,Polym. Degrad. Stab.,74, 151 (2001).
C. Peniche, E. Carlos, and JS. Roman,Polymer,39, 6549 (1998).
F. A. A. Tirkistani,Polym. Degrad. Stab.,60, 67 (1998).
K. Sreenivasan,Polym. Degrad. Stab.,52, 85 (1996).
T. Ikejima, K. Yogi, and Y. Inonu,Macromol. Chem. Phys.,200, 413 (1999).
X. Qu, A. Wirsen, and A. Albertsson,Polymer,41, 4841 (2000).
P. Gijsman, R. Steenbakkers, C. Furst, and J. Kersjes,Polym. Degrad. Stab.,78, 219 (2002).
J. González, C. Albano, R. Sciamanna, M. Ichazo, C. Rosales, J. Martînez, and M. Candal,Polym. Degrad. Stab.,68, 9 (2000).
T. Ozawa,Bull. Chem. Soc. Japan,38, 1881 (1965).
T. Ozawa,J. Thermal. Anal.,7, 601 (1975).
V. Gonzalez, C. Guerrero, and U. Ortiz,J. Appl. Polym. Sci.,78, 850 (2000).
G. Cardenenas, J. C. Paredes, G. Cabrea, and P. Casals,J. Appl. Polym. Sci.,86, 2742 (2002).
P. C. Carlos, A. M. Waldo, and J. S. Romãn,Polym. Degrad. Stab.,39, 21 (1993).
E. S. Kim, S. H. Kim, and Y. M. Lee,J. Polym., Part B: Polym. Phys. Ed.,34, 2367 (1996).
N. M. Langer and C. A. Wilkie,Polym. Adv. Technol.,9, 290 (1998).
D. A. Costa and C. M. F. Oliveira,J. Appl. Polym. Sci.,81, 2556 (2001).
H. W. Starkweather, Jr. R. John, and E. I. Barkley,J. Polym. Sci.: Polym. Phys. Ed.,19, 1211 (1981).
I. Garcia, C. Peniche, and J. M. Nieto,J. Thermal Anal.,21, 189 (1983).
H. Bockhorn, A. Horung, U. Horung, and J. Weichmann,Thermochimica Acta,337, 97 (1999).
Z. Czégény, E. Jakab, and M. Blazsó,Macromol. Mater. Eng.,287, 277 (2002).
K. Fukatsu,Polym. Degrad. Stab.,75, 479 (2002).
M. G. Lu, J. Y. Lee, M. J. Shim, and S. W. Kim,J. Appl. Polym. Sci.,85, 2552 (2001).
M. G. Lu, M. J. Shim, and S. W. Kim,J. Appl. Polym. Sci.,75, 1514 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liao, SK., Hung, CC. & Lin, MF. A kinetic study of thermal degradations of chitosan/polycaprolactam blends. Macromol. Res. 12, 466–473 (2004). https://doi.org/10.1007/BF03218428
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03218428