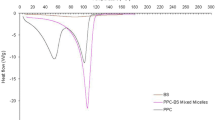
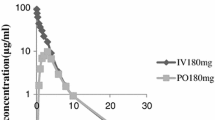
Summary
The aim of ths study was to investigate the pharmacokinetics of cefotaxime sodium (CEF) pharmacokinetics after oral application in the form of sodium 3α,7α-dihydroxy-12-keto-5β-cholanate (MKC) microvesicles (MV) in rat. Thirty Male Wister rats were divided into six groups (n=5 per group). Groups were treated orally with: i. CEF (15 mg/kg) saline solution (15 mg/kg); ii. CEF (15 mg/kg) saline solution with MKC (2 mg/kg); iii. CEF saline solution mixed with blank microvesicles; iv. CEF (15 mg/kg) encapsulated in microvesicles with saline solution; v. CEF saline solution (15 mg/kg) mixed with blank MKC microvesicules; vi. CEF (15 mg/kg) encapsulated in MKC microvesicules with saline solution. Data were analyzed using noncompartmental model. CEF oral bioavailability was increased twofold when coadministered with MKC and when encapsulated in microvesicles and ninefold when encap-sulated in MKC microvesicles compared to the same CEF dose administered orally as saline solution. The increased bioavailability of CEF resulting from CEF encapsulation in microvesicules with MKC suggests that this formulation can extend the application of CEF from parenteral only to oral application.
Similar content being viewed by others
References
Rosseel M.T., Vandewoude K.H., (2004): Liquid chromatographic determination of the plasma concentrations of cefotaxime and desacetylcefotaxime in plasma of critically ill patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 811, 159–163.
USP NF — United States Pharmacopeia and National Formulary, United States Pharmacopeial Convention Inc., Rock-ville, MD, USA, 26th Edition, 2003
Sharma P., Varma M.V., Chawla H.P., Panchagnula R. (2005): Absorption enhancement, mechanistic and toxicity studies of medium chain fatty acids, cyclodextrins and bile salts as peroral absorption enhancers. Farmaco, 6, 884–893.
Berge S.M., Henderson N.L., Frank M.J. (1983): Kinetics and mechanism of degradation of cefotaxime sodium in aqueous solution. J. Pharm. Sci., 72, 59–63.
Das Gupta V. (1984): Stability of cefotaxime sodium as determined by high-performance liquid chromatography. J. Pharm. Sci., 73, 565–567.
Fabre H., Eddine N.H., Berge G. (1984): Degradation kinetics in aqueous solution of cefotaxime sodium, a third-generation cephalosporin. J. Pharm. Sci., 73,611–618.
Esmieu F., Guibert J., Rosenkilde H.C., Ho I., Le Go A. (1980): Pharmacokinetics of cefotaxime in normal human volunteers. J. Antimicrob. Chemother., 6 Suppl A, 83–92.
Wise R., Wright N., et al. (1981): Pharmacology of cefotaxime and its desacetyl metabolite in renal and hepatic disease. Antimicrob. Agents Chemother., 19, 526–531.
Mesiha M.S., Ponnapula P., Plakogiannis F. (2002): Oral absorption of insulin encapsulated in artificial chyles of bile alts, palmitic acid and alpha tocoferol dispersions. Int. J. Pharm., 249, 1–5.
Michael S., Thole M., Dillman R., Fahr A., Drewe J., Fricker F. (2000):Improvement of intestinal absorption by synthetic bile acid derivative, cholylsarcosine. Eur. J. Pharm. Sci., 10, 133–140.
Mikov M., Al-Salami H., Golocorbin-Kon S., Skrbic R., Raskovic A., Fawcett J.P. (2008): The influence of 3α,7α-dihydroxy-12-oxo-5β-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur. J. Drug Metabol. Pharmacokinet., 33, 137–142.
Mrestani Y., Bretschneider B., Hard A., Neubert R.H.H. (2003): In vitro and in vivo studies of cefiprom using bile salts as absorption enhancers. J. Pharm. Pharmacol., 55, 1601–1606.
Mikov M., Raskovic A., Jakovljevic E., Dudvarski D., Fawcett J.P. (2005): Influence of the bile salt sodium 3α,7α-dihydroxy-12-oxo-5β-cholanate on ampicillin pharmacokinetics in rats. Asian J. Drug Metabol. Pharmacokinet., 5, 197–200.
Mikov M., Kevresan S., Kuhajda K., Jakovljevic V., Vasovic V. (2004): 3α,7α-dihydroxy-12-oxo-5β-cholanate as blood-brain barrier permeator. Pol. J. Pharmacol., 56, 367–371.
Sharma P., Chawla H.P., Panchagnula R. (2002): LC determination of cephalosporins in in vitro rat intestinal sac absorption model. J. Pharm. Biomed. Anal., 27, 39–50.
Ling S.S.N., Magrosso E., Khan N.A. K., Yen K.H. (2006): Enhanced oral bioavailability and intestinal lymphatic transport of hydrophilic drug using liposomes. Drug Dev. Ind. Pharm., 32, 335–345.
Miljkovic D., Kuhajda K., Hranisavljevic J. (1996): Selective C-12 oxidation of cholic acid. J. Chem. Res.; Suppl, 106–107.
Russell D.G., Alexander J. (1988): Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J. Immunol., 140, 1274–1279.
Ling S.S.N., Yuen K.H., Barker S.A. (2002): Simple Liquid chromatographic method for determination of cefotaxime in human and rat plasma. J. Chrom. B, 783, 297–301.
Hsieh D. (ed). Drug Permeation Enhancement Theory and Applications. Marcel Dekker Inc, New York, 1994
Mikov M., Fawcett J.P. (editors). In: (Mikov M., Fawcett J.P., Kuhajda K., Kevresan S., Kandrac J., eds) Bile acids: Chemistry, biosynthesis, analysis, chemical and metabolic transformations. Mediset Publisher, Geneva, 2007.
Alberts B., Bary D., Lewis J., Raff M., Roberts K., Watson J. (eds): Molecular Biology of the Cell. Garland, New York, 1983
Bretschneider B., Brandsch M., Neubert R. (1999): Intestinal transport of beta-lactam antibiotics: Analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm. Res., 16, 55–61.
Van Bambeke F., Michot J-M., Tulkens P.M. (2003): Antibiotic efflux pumps in eucariotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodimamics. J. Antimicrob. Chemother., 51, 1067–1077.
Maincent P., Le Verge R., Sado P., Couvreur P., Devissaquet J.P. (1986): Disposition kinetics and oral bioavailability of vincamine-loaded polyalkyl cyanoacrylate nanoparticles. J. Pharm. Sci., 75, 955–958.
Iwanaga K., Ono S., Narioka K., Kakemi M., Morimoto K., Yamashita S., Namba Y., Oku N., (1999): Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome’s surface on the GI transit of insulin. J. Pharm. Sci., 88, 248–252.
Florence A.T. (1997): The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm. Res., 14, 259–266.
Chen H., Langer R. (1988): Oral particulate delivery: status and future trends. Adv. Drug Deliv. Rev., 34, 339–350.
LeFevre M.E., Vanderhoff J.W., Laissue J.A., Joel D.D. (1978): Accumulation of 2-micron latex particles in mouse Peyer’s patches during chronic latex feeding. Experientia, 34, 120–122.
Lefevre M.E., Joel D.D., Schidlovski G. (1985): Retention of ingested latex particles in Peyer’s patches of germfree and conventional mice. Proc. Soc. Exp. Biol. Med., 179, 522–528.
LeFevre M.E., Joel D.D. (1986): Distribution of label after intragastric administration of 7Be-labeled carbon to weanling and aged mice. Proc. Soc. Exp. Biol. Med., 182, 112–119.
Jani P., Haibert G.W., Langridge J., Florence A.T. (1989): The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J. Pharm. Pharmacol., 41, 809–812.
Aramaki Y., Tomizawa H., Hara T., Yachi H., Tsuchiya S. (1993): Stability of liposomes in vitro and their uptake by rat Peyer’s patches following oral administration. Pharm. Res., 10, 1228–1231.
Senior K. (2001): Bilosomes: the answer to oral vaccine delivery? Drug Disco Today, 6, 1031–1032.
Pappo J. (1989): Generation and characterization of monoclonal antibodies recognizing follicle epithelial M cells in rabbit gut-associated lymphoid tissues. Cell. Immunol. 120: 31–41.
Maury J., Nicoletti C., Guzzo-Chambraud L., Maroux S. (1995): The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. Eur. J. Biochem., 228, 323–31.
Frey A., Giannasca K.T., Weltzin R.G., Giannasca P.J., Reggio H., Lencer W.I., Neutra M.R. (1996): Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J. Exp. Med., 184, 1045–1059.
Neutra M.R. (1998): Current concepts in mucosal immunity. V Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am. J. Physiol., 274,785–791.
Walde P., Sunamoto J., O’Connor J. (1987): The mechanism of liposomal damage by taurocholate. Biochim. Biophys. Acta, 905, 30–38.
O’Connor C.J., Wallace R.G., Iwamoto K., Taguchi T., Sunamoto J. (1985): Bile salt damage of egg phosphatidylcholine liposomes. Biochim. Biophys. Acta, 817, 95–102.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Golocorbin-Kon, S., Mikov, M., Arafat, M. et al. Cefotaxime pharmacokinetics after oral application in the form of 3α,7α-dihydroxy-12-keto-5β-cholanate microvesicles in rat. Eur. J. Drug Metabol. Pharmacokinet. 34, 31–36 (2009). https://doi.org/10.1007/BF03191381
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03191381