Abstract
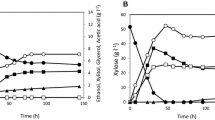
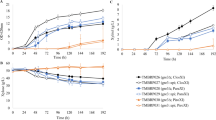
Efficient xylose utilisation by microorganisms is of importance to the lignocellulose fermentation industry. The aim of this work was to develop constitutive catabolite repression mutants in a xylose-utilising recombinantSaccharomyces cerevisiae strain and evaluate the differences in xylose consumption under fermentation conditions.S. cerevisiae YUSM was constitutively catabolite repressed through specific disruptions within theMIG1 gene. The strains were grown aerobically in synthetic complete medium with xylose as the sole carbon source. Constitutive catabolite repressed strain YCR17 grew four-fold better on xylose in aerobic conditions than the control strain YUSM. Anaerobic batch fermentation in minimal medium with glucose-xylose mixtures and N-limited chemostats with varying sugar concentrations were performed. Sugar utilisation and metabolite production during fermentation were monitored. YCR17 exhibited a faster xylose consumption rate than YUSM under high glucose conditions in nitrogen-limited chemostat cultivations. This study shows that a constitutive catabolite repressed mutant could be used to enhance the xylose consumption rate even in the presence of high glucose in the fermentation medium. This could help in reducing fermentation time and cost in mixed sugar fermentation.
Similar content being viewed by others
References
Ausubel F.M., Brent R., Kingson R.E., Moore D.D., Seidman J.G., Smity J.A., Struhl K. (1995). Gurrent Protocols in Molecular Biology. John Wiley and Sons, New York, USA.
Belinchon M.M., Gancedo J.M. (2003). Xylose and some nonsugar carbon sources cause catabolite repression inSaccharomyces cerevisiae. Arch. Microbiol., 180: 293–297.
Bruinenberg P.M., de Bot P.H.M., van Dijken J.P., Scheffers W.A. (1983). The role of redox balances in the anaerobic fermentation of xylose by yeasts. Appl. Microbiol. Biotechnol., 18: 287–292.
Busturia A., Lagunas R. (1986). Catabolite inactivation of the glucose transport system inSaccharomyces cerevisiae. J. Gen. Microbiol., 132: 379–385.
Chakravorty M., Viega L.A., Bacila M., Horecker B.L. (1962). Pentose metabolism in CandidaII. The diphosphoridine nucleotide-specific polyol dehydrogenase ofCandida utilis. J. Biol. Chem., 237: 1014–1020.
Chiang C., Knight S.G. (1960). Metabolism of D-xylose by moulds. Nature, 168: 79–81.
Chiang L.C., Gong C.S., Chen L.F., Tsao G.T. (1981). D-Xylulose fermentation to ethanol bySaccharomyces cerevisiae. Appl. Environ. Microbiol., 42: 284–289.
Christensen L.H., Schulze U., Nielsen J., Villadsen J. (1995). Acoustic off-gas analyzer for bioreactors: precision, accuracy and dynamics of detection. Chem. Engg. Science, 50: 2601–2610.
Eliasson A., Christensson C., Wahlborn C.F., Hahn-Hägerdal B. (2000). Anaerobic xylose fermentation by recombinantSaccharomyces cerevisiae carryingXYL1, XYL2, andXKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol., 66: 3381–3386.
Fiaux J., Çakar Z.P., Sonderegger M., Wüthrich K., Szyperski T., Sauer U. (2003). Metabolic-flux profiling of the yeastsSaccharomyces cerevisiae andPichia stipitis. Eukaryot. Cell, 2: 170–180.
Gancedo J.M. (1998). Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev., 62: 334–361.
Gardonyi M., Jeppsson M., Liden G., Gorwa-Grauslund M.F., Hahn-Hägerdal B. (2003). Control of xylose consumption by xylose transport in recombinantSaccharomyces cerevisiae. Biotechnol. Bioengg., 82: 818–824.
Gietz D., St. Jean A., Woods R.A., Schiestl R.H. (1992). Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res., 20: 1425.
Gong C.S., Claypool T.A., McCracken L.D., Mauw C.M., Ueng P.P., Tsao G.T. (1983). Conversion of pentoses by yeasts. Biotechnol. Bioengg., 15: 85–102.
Hahn-Hägerdal B., Hallborn J., Jeppsson H., Olsson L., Skoog K., Walfridsson M. (1993). Pentose fermentation to alcohol. In: Saddler, J.N., Ed., Bioconversion of forest and agricultural plant residues. CAB International, Wallingford, pp. 231–290.
Hahn-Hägerdal B., Wahlborn C.F., Gardonvi M., van Zyl W.H., Cordero Otero R.R., Jonsson L.J. (2001). Metabolic engineering ofSaccharomyces cerevisiae for xylose utilisation. Adv. in Biochem. Engg. Biotechnol., 73: 53–84.
Hamacher T., Johansson B., Gardonyi M., Hahn-Hägerdal B., Boles E. (2002). Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilisation. Microbiology, 148: 2783–2788.
Hayn M., Steiner W., Klinger R., Steinmuller H., Sinner M., Esterbauer H. (1993). Basic research and pilot studies on the enzymatic conversion of lignocellulosics. In: Saddler, J.N., Ed., Bioconversion of forest and agricultural plant residues. CAB International, Wallingford, pp. 33–72.
Ho N.W.Y., Chen Z., Brainard A.P. (1998). Genetically engineeredSaccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol., 64: 1852–1859.
Hochster R.M., Watson R.W. (1954). Enzymatic isomerisation of D-xylose to D-xylulose. Arch. Biochem., 48: 120–129.
Inoue H., Nojima H., Okayama H. (1990). High efficiency transformation ofEscherichia coli with plasmids. Gene, 96(1): 23–28.
Jeffries T.W., Jin Y.S. (2004). Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol., 63: 495–509.
Jeppsson M., Johansson B., Jensen P.R., Hahn-Hägerdal B., Gorwa-Grauslund M.F. (2003). The level of glucose-6-phosphate dehydrogenase activity strongly influences xylose fermentation and inhibitor sensitivity in recombinantSaccharomyces cerevisiae strains. Yeast, 20: 1263–1272.
Jin Y.S., Jones S., Shi N.Q., Jeffries T.W. (2002). Molecular cloning ofXYL3 (D-xylulokinase) fromPichia stipitis and characterization of its physiological function. Appl. Environ. Microbiol., 68: 1232–1239.
Jin Y.S., Ni H., Laplaza J.M., Jeffries T.W. (2003). Optimal growth and ethanol production from xylose by recombinantSaccharomyces cerevisiae require moderate D-xylulokinase activity. Appl. Environ. Microbiol., 69: 495–503.
Johansson B., Christensson C., Hobley T., Hahn-Hägerdal B. (2001). Xylulokinase overexpression in two strains ofSaccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl. Environ. Microbiol., 67: 4249–4255.
Johansson B., Hahn-Hägerdal B. (2002). The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose inSaccharomyces cerevisiae TMB3001. FEMS Yeast Res., 2(3): 277–282.
Kaniak A., Xue Z., Macool D., Kim J.H., Johnston M. (2004). Regulatory network connecting two glucose signal transduction pathways inSaccharomyces cerevisiae. Eukaryot. Cell, 3: 221–231.
Karhumaa K., Hahn-Hägerdal B., Gorwa-Grauslund M.F. (2005). Investigation of limiting metabolic steps in the utilisation of xylose by recombinantSaccharomyces cerevisiae using metabolic engineering. Yeast, 22: 359–368.
Karhumaa K., Wiedemann B., Hahn-Hägerdal B., Boles E., Gorwa-Grauslund M.F. (2006). Co-utilisation of L-arabinose and D-xylose by laboratory and industrialSaccharomyces cerevisiae strains. Microb. Cell Factories, 5:18.
Klein C.J.L., Olsson L., Nielsen J. (1998). Nitrogen-limited continuous cultivations as a tool to quantify glucose control inSaccharomyces cerevisiae. Enzyme Microb. Technol., 23: 91–100.
Kötter P., Ciriacy M. (1993). Xylose fermentation bySaccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 38: 776–783.
Kotyk A. (1967). Properties of the sugar carrier in baker’s yeast. 2. Specificity of transport. Folia Microbiol., 12: 121–131.
Lee J. (1997). Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol., 56: 1–24.
Lee W.J., Kim M.D., Ryu Y.W., Bisson L.F., Seo J.H. (2002). Kinetic studies on glucose and xylose transport inSaccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 60: 186–191.
Lidèn G., Persson A., Gustafsson L., Niklasson C. (1995). Energetics and product formation bySaccharomyces cerevisiae grown in anaerobic chemostats under nitrogen limitation. Appl. Microbiol. Biotechnol., 43: 1034–1038.
Lillie S., Pringle J.R. (1980). Reserve carbohydrate metabolism inSaccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol., 143: 1384–1394.
Meinander N.Q., Hahn-Hägerdal B. (1997). Influence of cosubstrate concentration on xylose conversion by recombinant,XYL1-expressingSaccharomyces cerevisiae: a comparison of different sugars and ethanol as cosubstrates. Appl. Environ. Microbiol., 63: 1959–1964.
Meinander N.Q., Boels I., Hahn-Hägerdal B. (1999). Fermentation of xylose/glucose mixtures by metabolically engineeredSaccharomyces cerevisiae strains expressingXYL1 andXYL2 fromPichia stipitis with and without overexpression ofTAL1. Bioresource Technol., 68: 79–87.
öhgren K., Bengtsson O., Gorwa-Grauslund M.F., Galbe M., Hahn-Hägerdal B., and Zacchi G. (2006). Simultaneous saccharification and co-fermentation of glucose and xylose in steam-pretreated corn stover at high fiber content withSaccharomyces cerevisiae TMB3400. J. Biotechnol., 126: 488–498.
Östling J., Carlberg M., Ronne H. (1996). Functional domains in the Mig1 repressor. Mol. Cell Biol., 16: 753–761.
Parrou J.L., François J. (1997). A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem., 248: 186–188.
Panek A.D. (1991). Storage carbohydrates. In: Rose A.H., Harrison J.S. Eds., The Yeasts. Vol. 4, 2nd edn., Academic Press, San Diego. pp. 655–678.
Richard P., Toivari M.H., Penttila M. (2000). The role of xylulokinase inSaccharomyces cerevisiae xylulose catabolism. FEMS Microbiol. Lett., 190: 39–43.
Roels J.A. (1983). Energetics and kinetics in biotechnology. 1st edn. Elsevier Biomedical Press BV, Amsterdam, The Netherlands
Roca C., Haack M.B., Olsson L. (2004). Engineering of carbon catabolite repression in recombinant xylose fermentingSaccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 63: 578–583.
Rose A.H., Vijayalakshmi G. (1993). Baker’s yeast. In: Rose, A.H., Harrison, J.S., Eds., Yeast Technology Vol. 5. Academic Press, San Diego, pp. 357–397.
Senac T., Hahn-Hägerdal B. (1990). Intermediary metabolite concentrations in xylulose- and glucose-fermentingSaccharomyces cerevisiae cells. Appl. Environ. Microbiol., 56: 120–126.
Sherman F., Fink G., Hicks J.B. (1983). Methods in yeast genetics. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, USA.
Sonderegger M., Sauer U. (2003). Evolutionary engineering ofSaccharomyces cerevisiae for anaerobic growth on xylose. Appl. Environ. Microbiol., 69: 1990–1998.
Sonderegger M., Jeppsson M., Hahn-Hägerdal B., Sauer U. (2004). Molecular basis for anaerobic growth ofSaccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis. Appl. Environ. Microbiol., 70: 2307–2317.
Thomsson E., Gustafsson L., Larsson C. (2005). Starvation response ofSaccharomyces cerevisiae grown in anaerobic nitrogen- or carbon-limited chemostat cultures. Appl. Environ. Microbiol., 71: 3007–3013.
Toivari M.H., Aristidou A., Ruohonen L., Penttila M. (2001). Conversion of xylose to ethanol by recombinantSaccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metabol. Engg., 3: 236–249.
Verduyn C., Postma E., Scheffers W.A., van Dijken J.P. (1992). Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast, 8: 501–517.
Verho R., Londesborough J., Penttila M., Richard P. (2003). Engineering redox cofactor regeneration for improved pentose fermentation inSaccharomyces cerevisiae. Appl. Environ. Microbiol., 69: 5892–5897.
Wahlbom C.F., Eliasson A., Hahn-Hägerdal B. (2001). Intracellular fluxes in a recombinant xylose-utilisingSaccharomyces cerevisiae cultivated anaerobically at different dilution rates and feed concentrations. Biotechnol. Bioengg., 72: 289–296.
Wahlborn C.F., van Zyl W.H., Jonsson L.J., Hahn-Hägerdal B., Cordero Otero R.R. (2003a). Generation of the improved recombinant xylose-utilisingSaccharomyces cerevisiae TMB 3400 by random mutagenesis and physiological comparison withPichia stipitis CBS 6054. FEMS Yeast Res., 3: 319–326.
Wahlbom C.F., Cordero Otero R.R., van Zyl W.H., Hahn-Hägerdal B., Jonsson L.J. (2003b). Molecular analysis of aSaccharomyces cerevisiae mutant with improved ability to utilise xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl. Environ. Microbiol., 69: 740–746.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thanvanthri Gururajan, V., Gorwa-Grauslund, MF., Hahn-Hägerdal, B. et al. A constitutive catabolite repression mutant of a recombinantSaccharomyces cerevisiae strain improves xylose consumption during fermentation. Ann. Microbiol. 57, 85–92 (2007). https://doi.org/10.1007/BF03175055
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03175055