Abstract
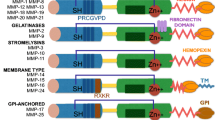
Matrix metalloproteinases are a family of zinc-containing proteolytic enzymes that break down extracellular matrix proteins in normal physiological processes such as embryogenesis, tissue growth, and wound healing. The family includes collagenases, gelatinases, stromelysins and metalloelastase. Observational and experimental data from studies of human malignancy indicate that these proteinases are induced by the tumour in order to reconstruct adjacent normal tissue to allow neovascularisation, tumour growth and spread. Tumours have been shown to overexpress certain matrix metalloproteinases relative to normal tissue and recent studies have shown an association between high levels of expression and poor prognosis. A large series of synthetic inhibitors have been developed using the structure of a principal substrate, collagen. The inhibitors contain a chemical group that binds the zinc atom in the active site of the metalloenzyme. Inhibition is specific for the known matrix metalloproteinase family and is reversible. Studies with these inhibitors and native tissue inhibitors of matrix metalloproteinases have shown that they can prevent the growth and spread of experimental tumours. In other studies, the inhibitors have been shown to be directly anti-angiogenic. Synthetic matrix metalloproteinase inhibitors have now reached the stage of clinical testing and preliminary results indicate that the compounds may be effective in slowing tumour growth. Trials currently underway should reveal whether this approach will become a standard part of anti-neoplastic therapy in the future.
Similar content being viewed by others
References
Taylor, A.C., Levy, B.M. and Simpson, J.W. (1970) Collagenolytic activity of sarcoma tissues in culture.Nature 228, 366–7.
Dresden, M.H., Heilman, S.A. and Schmidt, J.D. (1972) Collagenolytic enzymes in human neoplasms.Cancer Res. 32, 993–6.
Yamanishi, Y., Maeyens, E., Dabbous, M.K., Ohyama, H. and Hashimoto, K. (1973) Collagenolytic activity in malignant melanoma.Cancer Res. 33, 2790–801.
Liotta, L.A., Tryggvason, S., Garbisa, S., Hart, I., Foltz, C.M. and Shafie, S. (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen.Nature 284, 67–8.
Liotta, L.A., Tryggvason, K., Garbisa, S., Robey, P.G. and Abe, S. (1981) Partial purification and characterisation of a neutral protease which cleaves type IV collagen.Biochemistry 20, 100–4.
Wilhelm, S.C, Eisen, A.Z., Teter, M., Clark, S.D., Kronberger, A. and Goldberg, G. (1986) Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis.Proc. Natl. Acad. Sci. USA 83, 3756–60.
Hasty, K.A., Pourmotabbed, T.F., Goldberg, G.I., Thompson, J.P., Spinella, D.G., Stevens, R.M. and Mainardi, C.L. (1990) Human neutrophil collagenase; a distinct gene product with homology to other matrix metalloproteinases.J. Biol Chem. 265, 11421–4.
Collier, I.E., Wilhelm, S.M., Eisen, A.Z., Manner, B.L., Grant, G.A., Seltzer, J.L., Kronberger, A., He, C., Bauer, E.A. and Goldberg, G.I. (1988) H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metallo-protease capable of degrading basement membrane collagen.J. Biol Chem. 263, 6579–87.
Wilhelm, S.M., Collier, I.E., Manner, B.L., Eisen, A.Z., Grant, G.A. and Goldberg, G.I. (1989) SV40-transformed human lung fibroblasts secrete a 92-kDa Type IV collagenase which is identical to that secreted by normal human macrophages.J. Biol. Chem. 264, 17213–21.
Senior, R.M., Griffin, G.L., Fliszar, C.J., Shapiro, S.D., Goldberg, G.I. and Welgus, H.G. (1991) Human 92- and 72-kilodalton type IV collagenases are elastases.J. Biol. Chem. 266, 7870–5.
Wilhelm, S.M., Collier, I.E., Kronberger, A., Eisen, A.Z., Manner, B.L., Grant, G.G., Bauer, E.A. and Goldberg, G.I. (1987) Human skin fibroblast stromelysin: Structure, glycosylation, substrate specificity, and differential expression in normal and tumourigenic cells.Proc. Natl. Acad. Sci. USA 84, 6725–9.
Muller, D., Quantin, B., Gesnel, M.C., Millon-Collard, R., Abecassis, J. and Breathnach, R. (1988) The collagenase gene family in humans consists of at least four members.Biochem. J. 253, 187–92.
Basset, P., Bellocq, J.P., Wolf, C, Stoll, I., Hutin, P., Limacher, J.M., Podhajcer, O.L., Chenard, M.P., Rio, M.C. and Chambon, P. (1990) A novel metalloproteinase gene specifically expressed in stromal cell of breast carcinomas.Nature 348, 699–704.
Pei, D., Majmudar, G. and Weiss, S.J. (1994) Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3.J. Biol. Chem. 269, 25849–55.
Quantin, B., Murphy, G. and Breathnach, R. (1989) Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members.Biochemistry 28, 5325–34.
Shapiro, S.D. Kobayashi, D.K. and Ley, T.J. (1993) Cloning and characterisation of a unique elastolytic metalloproteinase produced by human alveolar macrophages.J. Biol. Chem. 268, 23824–9.
Sato, H., Takino, T., Okada, Y., Cao, J., Shinagawa, A., Yamamoto, E. and Seiki, M. (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells.Nature 370, 61–5.
Will, H. and Hinzmann, B. (1995) cDNA sequence and mRNA tissue distribution of a novel human matrix metalloproteinase with a potential transmembrane segment.Eur. J. Biochem. 231, 602–8.
Takino, T., Sato, H., Shinagawa, A. and Seiki, M. (1995) Identification of the second membrane-type matrix metalloproteinase (MT-MMP2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family.J. Biol. Chem. 270, 23013–20.
Puente, X.S., Pendas, A.M., Llano, E., Velasco, G. and Lopez-Otin, C. (1996) Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma.Cancer Res. 56, 944–9.
Strongin, A., Collier, I., Bannikov, G., Marmer, B.L., Grant, G.A. and Goldberg, G.I. (1995) Mechanism of cell surface activation of 72 kDa type IV collagenase.J. Biol. Chem. 270, 5331–8.
Cossins, J., Dudgeon, T.J., Catlin, G., Gearing, A.J.H. and Clements, J.M. (1996) Identification of MMP-18, a putative novel human matrix metalloproteinase.Biochem. Biophys. Res. Commun. 228, 494–8.
Docherty, A.J.P., Lyons, A., Smith, B.J., Wright, E.M., Stephens, P.E. and Harris, T.J.R. (1985) Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity.Nature 318, 66–9.
Stetler-Stevenson, W.G., Krutzsch, H.C. and Liotta, L.A. (1989) Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family.J. Biol Chem. 264, 17374–8.
Apte, S.S., Mattel, M.G. and Olsen, B.R. (1994) Cloning of the cDNA encoding human tissue inhibitor of metallo-proteinases-3 (TIMP-3) and the mapping of the TIMP-3 gene to chromosome 22.Genomics 19, 86–90.
Greene, J., Wang, M.S., Liu, Y.L.E., Raymond, L.A., Rosen, C. and Shi, Y.N.E. (1996) Molecular cloning and characterisation of human tissue inhibitor of metalloproteinase 4.J. Biol Chem. 271, 30375–80.
Kleiner, D.J. and Stetler-Stevenson, W.G. (1993) Structural biochemistry and activation of matrix metalloproteinases.Curr. Op. Cell Biol. 5, 891–7.
Powell, W.C. and Matrisian, L.M. (1996) Complex roles of matrix metalloproteinases in tumour progression.Curr. Top. Microbiol. Immun. 213, 1–21.
van der Stappen, J.W.J., Hendriks, T. and Wobbes, T. (1990) Correlation between collagenolytic activity and grade of histological differentiation in colorectal tumours.Int. J. Cancer 45, 1071–8.
Hewitt, R.E., Leach, I.H., Powe, D.G., Clark, I.M., Cawston, T.E. and Turner, D.R. (1991) Distribution of collagenase and tissue inhibitor metalloproteinases (TIMP) in colorectal tumours.Int. J. Cancer 49, 666–72.
Murray, G.I., Duncan, M.E., O’Neil, P., Melvin, W.T. and Fothergill, J.E. (1996) Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer.Nature Med. 2, 461–2.
Newell, K.J., Witty, J.P., Rodgers, W.H. and Matrisian, L.M. (1994) Expression and localisation of matrix-degrading metalloproteinases during colorectal tumourigenesis.Mol. Carcin. 10, 199–206.
Gallegos, N.C., Smales, C, Savage, F.G., Hembry, R.M. and Boulos, P.B. (1995) The distribution of matrix metalloproteinases and tissue inhibitor of metalloproteinases in colorectal cancer.Surg. Oncol. 4, 21–9.
Levy, A.T., Cioce, V., Sobel, M.E., Garbisa, S., Grigioni, W.F., Liotta, L.A. and Stetler-Stevenson, W.G. (1991) Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma.Cancer Res. 51, 439–44.
Liabakk, N-B., Talbot, L, Smith, R.A., Wilkinson, K. and Balkwill, F. (1996) Matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9) type IV collagenases in colorectal cancer.Cancer Res. 56, 190–6.
Brown, P.D., Bloxidge, R.E., Stuart, N.S.A., Gatter, K.C. and Carmichael, J. (1993) Correlation between expression of activated 72-kDa gelatinase and tumour spread in non-small cell lung carcinoma.J. Natl. Cancer Inst 85, 574–8.
Emmert-Buck, M.R., Roth, M.J., Zhuang, Z., Campo, E., Rozhin, J., Sloane, B.F., Liotta, L.A. and Stetler-Stevenson, W.G. (1994) Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon-cancer samples.Am. J. Pathol. 145, 1285–90.
Zeng, Z.S. and Guillem, J.G. (1995) Distinct pattern of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 mRNA expression in human colorectal cancer and liver metastases.Br. J. Cancer 72, 575–82.
Zeng, Z.S., Huang, Y., Cohen, A.M. and Guillem, J.G. (1996) Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9.J. Clin. Oncol. 14, 3133–40.
Zucker, S., Lysik, R.M., Zarrabi, M.H. and Moll, U. (1993) Mr92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer.Cancer Res. 53,140–6.
Yoshimoto, M., Itoh, F., Yamamoto, H., Hinoda, Y., Imai, K. and Yachi, A. (1993) Expression of MMP-7 (Pump-1) mRNA in human colorectal cancers.Int. J. Cancer 54, 614–8.
Mori, M., Barnard, G.F., Mimori, K., Ueo, H., Akiyoshi, T. and Sugimachi, K. (1995) Overexpression of matrix metalloproteinase-7 mRNA in human colon carcinomas.Cancer 75, 1516–9.
Ishikawa, T., Ichikawa, Y., Mitsuhashi, M., Momiyama, N., Chishima, T., Tanaka, K., Yamaoka, H., Miyazakic, K., Nagashima, Y., Akitaya, T. and Shimada, H. (1996) Matrilysin is associated with progression of colorectal tumor.Cancer Lett. 107, 5–10.
Porte, H., Chastre, E., Prevot, S., Nordlinger, B., Empereur, S., Basset, P., Chambon, P. and Gespach, C. (1995) Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes.Int. J. Cancer 64, 70–5.
Zeng, Z., Cohen, A.M., Zhang, Z., Stetler-Stevenson, W.G. and Guillem, J.G. (1995) Elevated tissue inhibitor of metalloproteinase 1 RNA in colorectal cancer stroma correlates with lymph node and distant metastases.Clin. Cancer Res. 1, 899–906.
Kossakowska, A.E., Huchcroft, S.A., Urbanski, S.J. and Edwards D.R. (1996) Comparative analysis of the expression patterns of metalloproteinases and their inhibitors in breast neoplasia, sporadic colorectal neoplasia, pulmonary carcinomas and malignant non-Hodgkin’s lymphomas in humans.Br. J. Cancer 73, 1401–8.
Yoshiji, H., Gomez, D.E. and Thorgeirsson, U.P. (1996) Enhanced RNA expression of tissue inhibitor of metallo-proteinases-1 (TIMP-1) in human breast cancer.Int J. Cancer 69, 131–4.
Grignon, D.J., Sakr, W., Toth, M., Ravery, V., Angulo, J., Shamsa, F., Pontes, J.E., Crissman, J.C. and Fridman, R. (1996) High levels of tissue inhibitor of metalloproteinase-2 (TIMP-2) expression are associated with poor outcome in invasive bladder cancer.Cancer Res. 56, 1654–9.
Alderson, M.R., Hamlin, I. and Staunton, M.D. (1971) The relative significance of prognostic factors in breast carcinoma.Br. J. Cancer 25, 646–56.
Anastassiades, O.T. and Pryce, P.M. (1974) Fibrosis as an indication of time in infiltrating breast cancer and its importance in prognosis.Br. J. Cancer 29, 232–9.
Carlomagno, C., Perrone, F., Lauria, R., De Laurentiis, M., Gallo, C., Morabito, A, Pettinato, G., Panico, L., Bellelii, T., Apicella, A., Petrella, G., Bianco, A.R. and De Placido, S. (1995) Prognostic significance of necrosis, elastosis, fibrosis and inflammatory cell reaction in operable breast cancer.Oncology 52, 272–7.
Parham, D.M., Hagen, N. and Brown, R.A. (1992) Simplified method of grading primary carcinomas of the breast.J. Clin. Pathol. 45, 517–20.
Takei, H., Iino, Y., Horiguchi, J. and Yakoe, T. (1995) Immunohistochemical fibronectin staining pattern and prognosis in invasive breast carcinoma.Oncology 52, 106–11.
Halvorsen, T.B. and Seim, E. (1989) Association between invasiveness, inflammatory cell reaction, desmoplasia and survival in colorectal cancer.J. Clin. Pathol. 42, 162–6.
Yamauchi, H., Kikuchi, Y. and Kakizaki, K. (1995) Relation between the stromal volume and liver metastasis in ductal cell carcinoma of the pancreas.Tohoku J. Exp. Med. 177, 179–81.
Leek, R.D., Lewis, C.E., Whitehouse, R., Greenall, M., Clarke, J. and Harris, A.L. (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma.Cancer Res. 56, 4625–9.
Pupa, S.M., Bufalino, R., Invernizzi, A.M., Andreola, S., Rilke, F., Lombardi, L., Colnaghi, M.L. and Menard, S. (1996) Macrophage infiltrate and prognosis in c-erB-2-overexpressing breast carcinomas.J. Clin. Oncol. 14, 85–94.
Beckett, R.P., Davidson, A.H., Drummond, A.H., Huxley, P. and Whittaker, M. (1996) Recent advances in matrix metalloproteinase inhibitor research.Drug. Dev. Today 1, 16–26.
Gearing, A.J.H., Beckett, P., Christodoulou, M., Churchill, M., Clements, J., Davidson, A.H., Drummond, A.H., Galloway, W.A., Gilbert, R., Gordon, J.L., Leber, T.M., Mangan, M., Miller, K., Nayee, P., Owen, K., Patel, S., Thomas, W., Wells, G., Wood, L.M. and Woolley, K. (1994) Processing of tumour necrosis factor-7 precursor by metalloproteinases.Nature 370, 555–7.
Reich, R., Thompson, E.W., Iwamoto, Y., Martin, G.R., Deason, J.R., Fuller, G.C. and Miskin, R. (1988) Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells.Cancer Res. 48, 3307–12.
Schultz, R.M., Silberman, S., Persky, B., Bajkowski, A.S. and Carmichael, D.F. (1988) Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells.Cancer Res. 48, 5539–45.
Eccles, S.A., Box, G.M., Court, W.J., Bone, E.A., Thomas, W. and Brown, P.D. (1996) Control of lymphatic and hematogenous metastases of a rat mammary carcinoma by the matrix metalloproteinase inhibitor batimastat (BB-94).Cancer Res. 56, 2815–22.
Sledge, G.W., Qulali, M, Goulet R., Bone, E.A. and Fife, R. (1995) Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice.J. Natl. Cancer Res. 87, 1546–50.
DeClerck, Y.A., Perez, N., Shimada, H., Boone, T.C., Langley, K.E. and Taylor, S.M. (1992) Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases.Cancer Res. 52, 701–8.
Johnson, M.D., Kim, H.R., Chesler, L., Tsao-Wu, G., Bouck, N. and Polverini, P.J. (1994) Inhibition of angiogenesis by tissue inhibitor of metalloproteinase.J. Cell Physiol. 160, 194–202.
Taraboletti, G., Garofalo, A., Belotti, D., Drudis, T., Borsotti, P., Scanziani, E., Brown, P. and Giavazzi, R. (1995) Inhibition of angiogenesis and murine hemangioma growth by batimastat, a synthetic inhibitor of matrix metalloproteinases.J. Natl. Cancer Inst 87, 293–8.
Anderson, I.C., Shipp, M.A., Docherty, A.J.P. and Teicher, B.A. (1996) Combination therapy including a gelatinase inhibitor and cytotoxic agent reduces local invasion and metastasis of murine Lewis lung carcinoma.Cancer Res. 56, 715–20.
Beckett, R.P. (1996) Recent advances in the field of matrix metalloproteinase inhibitors (patent update).Exp. Opin. Ther. Patents 6, 1305–15.
Galardy, R.E., Cassabone, M.E., Giese, C, Gilbert, J.H., Lapierre, F., Lopez, H.et al. (1994) Low molecular weight inhibitors in corneal ulceration.Ann. N.Y. Acad. Sci. 732, 315–23.
Macaulay, V.M., O’Byrne, K.J., Saunders, M.P., Salisbury, A., Long, L., Gleeson, F., Ganesan, T.S., Harris, A.L. and Talbot, D.C. (1995) Phase I study of matrix metalloproteinase (MMP) inhibitor batimastat (BB-94) in patients with malignant pleural effusions.Br. J. Cancer 71, 11 (Abstract).
Primrose, J., Bleiberg, H., Daniel, F., Johnson, P., Mansi, J., Neoptolemos, J., Seymour, M. and Van Belle, S. (1996) A dose-finding study of marimastat, an oral matrix metalloproteinase inhibitor, in patients with advanced colorectal cancer.Ann. Oncol. 7, 35 (Abstract).
Poole, C, Adams, M., Barley, V., Graham, J., Kerr, D., Louviaux, I., Perren, T., Piccart, M. and Thomas, H. (1996) A dose-finding study of marimastat, an oral matrix metalloproteinase inhibitor, in patients with advanced ovarian cancer.Ann. Oncol 7, 68 (Abstract).
Millar, A. and Brown, P. (1996) 360 patient meta-analysis of studies of marimastat, a novel matrix metalloproteinase inhibitor.Ann. Oncol. 7, 123 (Abstract).
Parsons, S.L., Watson, S.A., Griffin, N.R. and Steele, R.J.C. (1996) An open phase I/II study of the oral matrix metalloproteinase inhibitor marimastat in patients with inoperable gastric cancer.Ann. Oncol. 7, 47 (Abstract).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, P.D. Matrix metalloproteinase inhibitors in the treatment of cancer. Med Oncol 14, 1–10 (1997). https://doi.org/10.1007/BF02990939
Issue Date:
DOI: https://doi.org/10.1007/BF02990939