Abstract
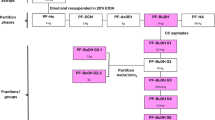
The chloroform extract of the aerial part ofLimnophila geoffrayi showed antimycobacterial and antioxidant activities. Bioassay-guided fractionation has led to the isolation of the flavones nevadensin (5,7-dihydroxy-6,8,4′-trimethoxyflavone,1) and isothymusin (6,7-dimethoxy-5,8,4′-trihydroxyflavone,2). Both compounds 1 and 2 exhibited inhibition activity againstMycobacterium tuberculosis, with equal MIC value of 200 μg/mL Only compound2 exhibited antioxidant activity against the radical scavenging ability of DPPH, with the IC50 value of 7.7 μg/mL The crude hexane, chloroform and methanol extracts as well as the pure compounds1 and2 did not exhibit mutagenic activity in theBacillus subtilis recassay.
Similar content being viewed by others
References
Barberan, F. A. T., Ferreres, F., and Tomas, F., TLC, UV and acidic treatment in the differentiation of 5,6-and 5,8-dihy-droxyflavones, 3-methoxyflavones and flavonols.Tetrahedron, 41, 5733–5740 (1985).
Barberan, F. A. T., Ferreres, F., Tomas, F., and Guirado, A., Electron impact mass spectrometric differentiation of 5,6-dihydroxy-7,8-dimethoxy- and 5,8-dihydroxy-6,7-dimethoxyflavones.Phytochemistry, 25, 923–925 (1986).
Brand-Williams, W., Cuvelier, M. E., and Berset, C., Use of a free radical method to evaluate antioxidant activity.Food Sci. Tecrtnol., 28, 25–30 (1995).
Bunyapraphatsara, N. and Chokechaijaroenpom, O., Faculty of Pharmacy, Mahidol Univercity and National Center for Genetic and Engineering and Biotechnology.Thai Medicinal Plants, 3, 53 (2000).
Collins, L. A. and Franzblau, S. G., Microplate Alamar Blue Assay versus BACTEC 460 system for high-throughput screening of compounds againstMycobacterium tuberculosis andMycobacterium avium.Antimicrob. Agents Chemother., 41, 1004–1009 (1997).
Farkas, L, Nogradi, M., Sudarsanam, V., and Herz, W., Constituents ofIva species. V. Isolation, structure, and synthesis of nevadensin, a new flavone fromIva nevadensis M. E. Jones andIva acerosa (Nutt.) Jackson.J. Org. Chem., 31, 3228–3231 (1966).
Ferreres, F., Barberan, F. A. T., and Tomas, F., 5,6,4′-Trihydroxy-7,8-dimethoxyflavone fromThymus membranaceus.Phytochemistry, 24, 1869–1871 (1985).
Greenham, J., Vassiliades, D. D., Harbome, J. B., Williams, C. A., Eagles, J., Grayer, R. J., and Veitch, N. C., A distinctive flavonoid chemistry for the anomalous genusBiebersteinia.Phytochemistry, 56, 87–91 (2001).
Horie, T., Kawamura, Y., Yamamoto, H., Kitou, T., and Yamashita, K., Synthesis of 5,8-dihydroxy-6,7-dimethoxyflavones and revised structures for some natural flavones.Phytochemistry, 39, 1201–1210 (1995).
Horie, T., Ohtsuru, Y., Shibata, K., Yamashita, K., Tsukayama, M., and Kawamura, Y.,13C-NMR spectral assignment of the A-ring of polyoxygenated flavones.Phytochemistry, 47, 865–874 (1998).
Horie, T., Shimoo, H., Masumura, M., and Okumura, S., Demethoxysudachitin.Nippon Kagaku Zasshi, 83, 602–604 (1962).
Kada, T., Tutikawa, K., and Sadaie, Y.,In vitro and host-mediated “rec-assay” procedures for screening chemical mutagens, and phloxine, a mutagenic red dye detected.Mutation Res., 16, 165–174 (1972).
Kelm, M. A., Nair, M. G., Strasburg, G. M., and DeWitt, D. L., Antioxidant and cyclooxygenase inhibitory phenolic compounds fromOcimum sanctum Linn.Phytomedicine, 7, 7–13 (2000).
Liu, M. C., Chen, Z. S., Chung, L. C., Yang, M. S., Ho, S. T., and Chen, M. T., Studies on hypotensive constituents ofLimnophila rugosa.Chung-hua Yao Hsueh Tsa Chih, 43, 35–40 (1991).
Saralamp, P. and Chuakul W., editors.Encyclopaedia of Medicinal Plants, Vol. 4: Kok Ya E-sarn (Medicinal Plants of the Northeast). Mahidol University Foundation, Bangkok, p. 159, (1999).
Wang, H., Nair, M. G., Strasburg, G. M., Booren, A. M., and Gray, J. I., Antioxidant polyphenols from tart cherries (Prunus cerasus).J. Agri. Food Chem., 47, 840–844 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suksamrarn, A., Poomsing, P., Aroonrerk, N. et al. Antimycobacterial and antioxidant flavones fromLimnophila geoffrayi . Arch Pharm Res 26, 816–820 (2003). https://doi.org/10.1007/BF02980026
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980026