Abstract
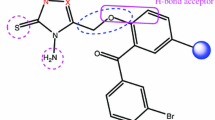
Dihydrofolate reductases (DHFR) of human,Candida albicans andE. coli were docked with their original ligands of X-ray crystal complex using QXP (Quick eXPIore), a docking program. Conditions to reproduce the crystal structures within the root mean square deviation (rmsd) of 2.00 Å were established. Applying these conditions, binding modes and species-specificities of a novel antibacterial compound, N4-(2-acetoxyethoxymethyl)-2-acetylpyridine thiosemicarbazone (AATSC), were studied. As the results, the docking program reproduced the crystal structures with average rmsd of six ligands as 0.91 A ranging from 0.49 to 1.45 ÅA. The interactions including the numbers of hydrogen bonds and hydrophobic interactions were the same as the crystal structures and superposition of the crystal and docked structures almost coincided with each other. For AATSC, the results demonstrated that it could bind to either the substrate or coenzyme sites of DHFR in all three species with different degrees of affinity. It confirms the experimentally determined kinetic behavior of uncompetitive inhibition against either the inhibitor or the coenzyme. The docked AATSC overlapped well with the original ligands and major interactions were consistent with the ones in the crystal complexes. The information generated from this work should be useful for future development of antibacterial and antifungal agents.
Similar content being viewed by others
References
Bohacek, R. S., Dalgarno, D. C., Hatada, M., Jacobsen, V. A., Lynch, B. A., Macek, K. J., Merry, T., Metcalf III, C. A., Narula, S. S., Sawyer, T. K., Shakespeare, W. C., Violette, S. M., and Weigele, M., X-ray structure of citrate bound to Src SH2 leads to a high-affinity, bone-targeted Src SH2 inhibitor.J. Med. Chem., 44, 660–663 (2001).
Bolin, J. T., Filman, D. J., Matthews, D. A., Hamlin, R.C., and Kraut, J., Crystal structures o.Escherichia coli andLactobacillus casei dihydrofolate reductase refined at 1.7 resolution.J. Biol. Chem., 257 (22), 13650–13662 (1982).
Bruice, R Y.,Organic Chemistry (fourth eds). Prentice Hall, New Jersey, (1995).
Bystroff, C., Oatley, S. J., and Kraut, J., Crystal structures o.Escherichia coli dihydrofolate reductase: the NADP+ holoen-zyme and the folate NADP+ ternary complex: Substrate binding and a model for the transition state.Biochemistry, 29, 3263–3277 (1990).
Cody, V., Luft, J. R., Ciszak, E., Kalman, T. I., and Freisham, J. H., Crystal structure determination at 2.3 of recombinant human dihydrofolate reductase ternary complex with NADPH and methotrexate-tetrazole.Anticancer Drug Design, 7, 483–491 (1992).
Cody, V., Wojtczak, A., Kalman, T. I., Freisham, J. H., and Blakley, R. L., Conformational analysis of human dihydrofolate reductase inhibitor complexes: Crystal structure determination of wild type and F31 mutant binary and ternary-inhibitor complexes. Ayling, J. E., Nair, M. G. and Baugh, C. M. (Eds.), InChemistry and Biology of Pteridines and Folates. Plenum Press, New York, pp. 48–486, (1993).
Cody, V., Galitsky, N., Luft, J. R., Pangborn, W., Gangjee, A., Devraj, R., Queener, S. F., and Blakley, R. L., Comparison of ternary complexes o.Pneumocystis carinii and wild type human dihydrofolate reductase with coenzyme NADPH and a novel classical antitumor furo[2,3-d]pyrimidine antifolate.Acta Crystallographica, D53, 638 (1997).
Cody, V., Galitsky, N., Luft, J. R., Pangborn, W., Blakley, R. L., and Gangjee, A., Comparison of ternary crystal complexes of F31 variants of human dihydrofolate reductase with NADPH and a classical antitumor furopyrimidine.Anticancer Drug Des., 13, 307–315 (1998).
Cocco, L., Roth, B., Temple, C. Jr., Montgomery, J. A., London, R. E., and Blakley, R. L., Protonated state of methotrexate, trimethoprim, and pyrimethamine bound to dihydrofolate reductase.Arch. Biochem. Biophys., 226, 567–577 (1983).
Fierke, C. A., Johnson, K. A., and Benkovic, S. J., Construction and evaluation of the kinetics scheme associated with dihydrofolate reductase fro.Escherichia coli.Biochemistry, 26, 4085–4092 (1987).
Foye, W. O., Banijamali, A. R. and Patarapanich, C., Synthesis and antimicrobial activities of N4-(2-acetoxyethoxymethyl) thiosemicarbazones and N3-(2-acetoxyethoxymethyl) thioureas.J. Pharm. Sci., 75, 1180–1184 (1986).
Foye, W. O., Dabade, S. V., Kelly, C. J., Lebrun, E., and van Rapenbusch, R., Synthesis and dihydrofolate reductase inhibitory activity of N4-2-L-glutaryl-N1-heteroaryl thiosemicarbazones.Med. Chem. Res., 8, 542–553 (1998).
Gokhale, V. M., and Kulkarni, V. M., Selectivity analysis of 5-(arylthio)-2,4-diaminoquinazolines as inhibitors of Candida albicans dihydrofolate reductase by molecular dynamics simulations. J. Comput. Aided Mol., Des. 14, 495–500 (2000).
Graffner-Nordberg, M., Marelius, J., Ohlsson, S., Persson, A., Swedberg, G., Anderson, P., Andersson, S. E., Aqvist, J., and Hallberg, A., Computational predictions of binding affinities to dihydrofolate reductase: Synthesis and biological evaluation of methotrexate analogues.J. Med. Chem., 43, 3852–3861 (2000).
Jacques, S. L., Ejim, L. J., and Wright, G. D., Homoserine dehy-drogenase from Saccharomyces cerevisiae: Kinetic mechanism and stereochemistry of hydride transfer.Biochim. Biophys. Acta, 1544, 42–54 (2001).
Lebrun, E., Tu, Y. X., van Rapenbusch, R., Banijamali, A. R., and Foye, W. O., Inhibition of bovine dihydrofolate reductase and enhancement of methotrexate sensitivity by N4-(2-acet-oxyethoxymethyl)-2-acetylpyridine thiosemicarbazone.Biochim. Biophys. Acta., 1034, 81–85 (1990).
McMartin, C., and Bohacek, R. S., QXP: Powerful, rapid computer algorithms for structure-based drug design.J. Comput. Aided Mol. Des., 11, 333–344 (1997).
Meiering, E. M., and Wagner, G., Detection of long-lived bound water molecules in complexes of human dihydrofolate reductase with methotrexate and NADPH.J. Mol. Biol., 247, 294–308 (1995a).
Meiering, E. M., Li, H., Delcamp, T. J., Freisheim, J. H., and Wagner, G., Contributions of tryptophan 24 and glutamate 30 to binding long-lived water molecules in the ternary complex of human dihydrofolate reductase with methotrexate and NADPH studied by site-directed mutagenesis and nuclear magnetic resonance spectroscopy.J. Mol. Biol., 247, 309–325 (1995b).
Metcalf III, C. A., Eyermann, C. J., Bohacek, R. S., Haraldson, C. A., Varkhedkar, V. M., Lynch, B. A., Bartlett, C., Violette, S. M., and Sawyer, T. K., Structure-based design and solid-phase parallel synthesis of phosphorylated nonpeptides to explore hydrophobic binding at the sre SH2 (Src SH2) domain.J. Comb. Chem., 2, 305–313 (2000).
Sawaya, M., and Kraut, J., Loop and subdomain movements in the mechanism of escherichia coli dihydrofolate reductase: Crystallographic evidence.Biochemistry, 36, 586–603 (1997).
Schweitzer, B. I., Dicker, A. P., and Bertino, J. R., Dihydrofolate reductase as a therapeutic target.FASEB J., 4, 2441–2452 (1990).
Shakespeare, W., Yang, M., Bohacek, R., Cerasoli, F., Stebbins, K., Sundaramoorthi, R., Azimioara, M., Vu, C., Pradeepan, S., Metcalf III, C., Haraldson, C., Merry, T., Dalgarno, D., Narula, S., Hatada, M., Lu, X., van Schravendijk, M. R., Adams, S., Violette, S., Smith, J., Guan, W., Bartlett, C., Herson, J., Luliucci, J., Weigele, M., and Sawyer, T., Structure-based design of an osteoclast-selective, nonpeptide Src homology 2 inhibitor with in vivo antiresorptive activity.Proc. Natl. Acad. Sci., 97, 9373–9378 (2000).
Stilz, H. U., Guba, W., Jablonka, B., Just, M., Klingler, O., Konig, W., Wehner, V., and Zoller, G., Discovery of an orally active non-peptide fibrinogen receptor antagonist based on the hydantoin scaffold.J. Med. Chem., 44, 1158–1176 (2001).
Vu, C. B., Corpuz, E. G., Merry, T. J., Pradeepan, S. G., Bartlett, C., Bohacek, R. S., Botfield, M. C., Eyermann, C. J., Lynch, B. A., MacNeil, I. A., Ram, M. K., van Schravendijk, M. R., Violette, S., and Sawyer, T. K., Discovery of potent and selective SH2 inhibitors of the tyrosine kinase ZAP-70.J. Med. Chem., 42, 4088–4098 (1999).
Weiner, S. J., Kollman, P. A., Case, D. A., Singh, U. C., Ghio, C., Algona, C., Profeta, S., and Weiner, P., A new force field for molecular mechanical simulation of nucleic acids and proteins.J. Am. Chem. Soc., 106, 765–784 (1984).
Whitlow, M., Howard, A. J., Stewart, D., Hardman, K. D., Kuyper, L. F., Baccanari, D. P., Fling, M. E., and Tansik, R. L., X-ray crystallographic studies of Candida albicans dihydro-folate reductase: High resolution structures of the holoenzyme and an inhibited ternary complex.J. Biol. Chem., 48, 30289–30298 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, I.H., Kim, C. Flexible docking of an acetoxyethoxymethyl derivative of thiosemicarbazone into three different species of dihydrofolate reductase. Arch Pharm Res 25, 807–816 (2002). https://doi.org/10.1007/BF02976996
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976996