Abstract
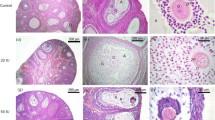
In adult rats, removal of one ovary leads to an acute albeit transient rise in serum follicle stimulating hormone and an increase in the weight of the remaining ovary. In an attempt to correlate the high titre of endogenous follicle stimulating hormone with the changes taking place at the macromolecular level, the phenomenon of compensatory ovarian hypertrophy was studied for one cycle after hemiovariectomy at metoestrus in the adult, cycling female rats derived from the Holtzman strain. The significant finding with respect to hormonal changes was an acute follicle stimulating hormone surge commencing 6h post-unilateral ovariectomy, reaching a maximum at 12 h and declining thereafter, hitherto not reported in the Holtzman strain. Serum luteinizing hormone, prolactin, oestradiol-17β and testosterone remained unaltered while progesterone showed a decline at 6 h after surgery. There was an increase in the number of healthy class III (> 350 µm) follicles with a concomitant drop in atretic class III follicles 24 h post-unilateral ovariectomy. Analysis for DNA, RNA and protein content showed that all three constituents registered a continuous rise in the hypertrophying ovary up to 120h after surgery. When expressed as ¼g/mg ovarian weight, the increase in DNA reached a maximum at 24 h and declined thereafter. The kinetics of DNA synthesis was followed by pulse labelling with [3H] thymidine at 18, 24, 36 and 48 h after unilateral ovariectomy. Maximum incorporation occurred at 36 h. Autoradiographic studies showed that the granulosa cells of healthy follicles preferentially incorporated the label. In an extension of this study, it was found that labelling index registered a significant increase following ovariectomy, the maximum being reached at 24 h especially in classIII follicles. The results clearly point out the crucial role of hyperplasia in the response of the contralateral ovary to the surgery and implicate the rise in follicle stimulating hormone as the primary signal for initiation of such a response. This raises the question whether in compensatory ovarian hypertrophy follicle stimulating hormone has a mitogenic role
Similar content being viewed by others
References
Abraham G E 1974 Radioimmunoassay of steroids in biological materials;Acta Endocrinol. Copenh. Suppl. 75 183 1–42
Ackland J F, D’Agostino J, Ringstrom S J, Hostetler J P, Mann B G and Schwartz N B 1990 Circulating radioimmunoassayable inhibin during periods of transient follicle-stimulating hormone rise: secondary surge and unilateral ovariectomy;Biol. Reprod. 43 347–352
Alvarez R D, Grizzle W E, Smith L J and Miller D M 1989 Compensatory ovarian hypertrophy occurs by a mechanism distinct from compensatory growth in the regenerating liver;Am. J. Obstet. Gynecol. 161 1653–1657
Bhagat L 1991 Compensatory ovarian hypertrophy and its significance for the characterization of pinealantigonadotrophic activity, Ph. D. thesis, University of Delhi, Delhi
Burton K 1956 A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid;Biochem. J. 62 315–323
Butcher R L 1977 Changes in gonadotropins and steroids associated with unilateral ovariectomy of the rat;Endocrinology 101830–840
Callantine M R, Humphrey R R and Lee S-L 1965 Effect of follicle-stimulating hormone on ovarian nucleic acid content;Endocrinology 76 332–334
Cunningham G R, Tindall D J, Huckins C and Means A R 1978 Mechanisms for the testicular hypertrophy which follows hemicastration;Endocrinology 102 16–23
Curry T E Jr, Lawrence I E Jr and Burden H W 1984 Effect of ovarian sympathectomy on follicular development during compensatory ovarian hypertrophy in the guinea pig;J. Reprod. Fertil. 71 39–44
Eshkol A and Pariente C 1984 Regulation of granulosa cell functions by cyclic AMP and cyclic GMP; in Hormone receptor in growth and reproduction (eds) B B Saxena, K J Catt, L Birnbaumer and L Martini (New York: Raven Press) Vol. 9, pp 141–148
Hirshfield A N 1982 Follicular recruitment in long-term hemicastrate rats;Biol. Reprod. 27 48–53
Hirshfield A N 1983 Compensatory ovarian hypertrophy in the long-term hemicastrate rat: size distribution of growing and atretic follicles;Biol. Reprod. 28 271–278
Hirshfield A N 1989 Rescue of atretic follicles invitro andin vivo;Biol. Reprod. 40 181–190
Howland B E and Skinner K R 1973 Effect of hemiovariectomy on serum FSH and LH levels during the oestrous cycle in the rat;J. Reprod. Fertil 32 501–503
Kopriwa B M and Leblond C P 1962 Improvements in the coating technique of radioautography;J. Histochem ·Cytochem. 10 269–284
Kumari M and Duraiswami S 1986 Protein synthesis in the rat seminiferous epithelium during onset of spermatogenesis: an autoradiographic study;Andrologia 18 474–482
Lipschutz A 1928 New developments in ovarian dynamics and the law of follicular constancy;Br. J. Exp. Biol 5 283–291
Lowry O H, Rosebrough N J, Farr A L and Randall R J 1951 Protein measurement with the Folin phenol reagent;J. Biol Chem. 193 265–275
McLaren A 1963 Mechanism of ovarian compensation following unilateral ovariectomy in mice;J. Reprod · Fertil 6 321–322
Midgley A R Jr 1966 Radioimmunoassay: a method for human chorionic gonadotropin and human luteinizing hormone;Endocrinology 79 10–18
Nance D W, White J P and Moger W H 1983 Neural regulation of the ovary: evidence for hypothalamic asymmetry in endocrine control;Brain Res. Bull 10 353–355
Orth J M, Higginbotham C A and Salisbury R L 1984 Hemicastration causes and testosterone prevents enhanced uptake of [3H] thymidine by Sertoli cells in testes of immature rats;Biol Reprod. 30 263–270
Otani T and Sasamoto S 1982 Plasma and pituitary hormone changes and follicular development after unilateral ovariectomy in cyclic rats;J. Reprod. Etėl. 65 347–353
Pedersen T and Peters H 1968 Proposal for a classification of oocytes and follicles in the mouse ovary;J. Reprod. Fertil. 17 555–557
Peluso J J and Steger R W 1978 Role of FSH in regulating granulosa cell division and follicular atresia in rats;J. Reprod. Fertil. 54 275–278
Peppler R D 1972 FSH and LH levels in the intact and unilaterally ovariectomizcd cycling rat;Acta Endocrinol.Copenh. 69 267–280
Peppler R D and Greenwald G S 1970 Influence of unilateral ovariectomy on follicular development in cycling rats;Am. J. Anat. 127 9–14
Peters H and Braathen B 1973 The efect of unilateral ovariectomy in the neonatal mouse on follicular development;J. Endocrinol. 56 85–89
Ramirez V D and Sawyer C H 1974 A sex difference in the rat pituitary FSH response to unilateral gonadectomy as revealed by plasma RIA;Endocrinology 94 475–482
Rao M C, Midgley A R Jr and Richards J S 1978 Hormonal regulation of ovarian cellular proliferation;Cell 14 71–78
Rogers A W 1979Practical autoradiography (Amersham: Amersham International Ltd.) pp 43–44
Ryle M 1971 Morphological responses to pituitary gonadotrophins by mouse ovaries in vitro;J. Reprod. Fertil. 20 307–312
Schmidt G 1957 Determination of nucleic acids by phosphorus analysis;Methods Enzymol. 3 671–679
Schneider W C 1957 Determination of nucleic acids in tissues by pentose analysis;Methods Enzymol. 3 680–684
Sheela Rani C S and Moudgal N R 1978 Studies on follicular growth in the immature rat and hamster: Effect of a single injection of gonadotropin or estrogen on the rale of 3H-thymidine incorporation into ovarian DNA in vitro;Proc. Indian Acad. Sci. (Exp. Biol.) B87 41–51
Sokal R R and Rohlf F J 1981 Two way analysis of variance; inBiometry, Second Edition (San Francisco: W H Freeman and Co.) pp 321–371
Welschen R, Dullaart J and de Jong F H 1978 Inter-relationships between circulating levels of estradiol-17β, progesterone, FSH and LH immediately after unilateral ovariectomy in the cyclic rat;Biol. Reprod 18 421–427
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhagat, L., Duraiswami, S. & Kumari, G.L. Role of follicle stimulating hormone in the induction of hyperplasia during compensatory ovarian hypertrophy. J Biosci 18, 59–72 (1993). https://doi.org/10.1007/BF02703038
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02703038