Abstract
Impedance data, e.g., system responses, from perturbing small amplitude applied sinusoid signals of near DC to high kilohertz frequencies, give chemical information. Analysis of frequency-dependent imaginary and real impedance proceeds from equivalent analog circuit elements to chemical and physical significance determined from many model systems. Already, it is possible to interpret bulk transport processes, surface kinetic effects, diffusion phenomena, and dependencies on the type of contacts: symmetric ion contact, symmetric metal contact or asymmetric metal-ion interfaces, and cell design; even (battery or sensor) and odd numbered (constrained junction or immiscible liquid) interfaces in a system. These analyses cover the chemical origins, locations and meanings of the lumped resistances, capacitances and transmission lines that are introduced by engineers in their strict analog interpretations of the impedance data.
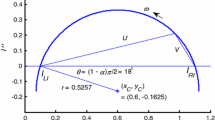
Examples cover simple ohmic, simple diffusive behavior, complex behavior with surface interfacial kinetics or surface resistances, and with finite (nonblocking) or infinite (blocking) DC impedance. High and low frequency responses may show socalled constant phase element character that suggests fractal behavior. Low frequency data occasionally appear in the second quadrant of impedance plane plots. These results are caused by negative capacitances and resistances.
In this paper, chemical interpretations of analog circuit elements are mainly based on theory and observations of thin cells of electrolytes and solid and liquid films (membranes) that are ionic or mixed ionic/electronic conductors. The information should carry over into thickened, gelled, and tissue electrolyte phases and serve as a basis for medically-oriented, perhaps diagnostic impedance measurement applications already pioneered by Herman Schwan.
Similar content being viewed by others
Abbreviations
- A :
-
active area
- a i :
-
ion activity
- a red :
-
reduced species activity
- a ox :
-
oxidized species activity
- C :
-
capacitance or capacitance/unit area
- C i :
-
ion concentration
- C red :
-
reduced species concentration
- C ox :
-
oxidized species concentration
- D :
-
diffusion coefficient
- d :
-
half film (membrane) thickness
- F :
-
Faraday constant
- J e :
-
electron flux
- J i :
-
ion flux
- n :
-
number of electrons in half cell reaction
- R :
-
resistance
- R :
-
gas constant appearing with T
- T :
-
absolute temperature
- u e :
-
electron mobility
- u i :
-
ion mobility
- Z(jω):
-
impedance
- z i :
-
ion charge
- z red :
-
reduced species charge
- z ox :
-
oxidized species charge
- α:
-
parameter of Cole-Cole plot
- ε:
-
dielectric constant
References
Armstrong, R.D. Impedance plane display for an electrode with diffusion restricted to a thin layer. J. Electroanal. Chem. 198:177–180; 1986.
Armstrong, R.D.; Lindholm, B.; Sharp, M. Impedance characteristics of a modified electrode. J. Electroanal. Chem. 202:69–74; 1986.
Bard, A.J.; Faulkner, L.R. Electrochemical methods. New York: John Wiley & Sons; 1980.
Berlouis, L.E.A.; Chatham, J.; Girault, H.H.J.; Schiffrin, D.J. Transport properties of phospholipid monolayer membrane models. Ext. Abstr. Electrochem. Soc. 83-1(620):935–936; 1983.
Brumleve, T.R.; Buck, R.P. Numerical solution of Nernst-Planck and Poisson equations systems: Applications to membrane electrochemistry and solid state physics. J. Electroanal. Chem. 90:1–31; 1978.
Brumleve, T.R.; Buck, R.P. Potential reversals across site-free, passive membranes—A simulation analysis. J. Electroanal. Chem. 126:55–72; 1981.
Brumleve, T.R.; Buck, R.P. Transmission line equivalent circuit models for electrochemical impedances. J. Electroanal. Chem. 126:73–104; 1981.
Buck, R.P. Transient electrical behavior of glass membranes. J. Electroanal. Chem. 18:363–399; 1968.
Buck, R.P. Potential-generating processes at interfaces: From electrolyte/metal and electrolyte/membrane to electrolyte/semiconductor. In: Cheung, P.W.; Fleming, D.G.; Neuman, M.R.; Ko, W.H., eds. Theory, design, and biomedical applications of solid state chemical sensors. W. Palm Beach: CRC Press, Inc.; 1978: pp. 1–41.
Buck, R.P. Transport properties of ionic conductors. Bergveld, P.; Zeme, J.; Middelhoek, S., eds; In: Chemically sensitive electronic devices. Amsterdam, Netherlands: Elsevier Pub. Co.; 1981: pp. 137–260.
Buck, R.P. The impedance methods applied to the investigation of ion-selective electrodes. Ion-Selective Electrode Rev. 4:3–74; 1982.
Buck, R.P. Electrochemistry of ion-selective electrodes. In: White, R.E.; Bockris, J.O.; Conway, B.E.; Yeager, E., eds. Comprehensive treatise of electrochemistry. New York: Plenum Pub. Co.; 1984: pp. 137–248.
Buck, R.P. Kinetics of bulk and interfacial ionic motion: Microscopic bases and limits for the Nernst-Planck equation applied to membrane systems. J. Membr. Sci. 17:1–62; 1984.
Buck, R.P. Diffusion-migration impedances for finite, one-dimensional transport in thin layer and membrane cells: An analysis of derived electrical quantities and equivalent circuits. J. Electroanal. Chem. 210:1–19; 1986.
Buck, R.P. Diffusion-migration impedances for finite, one-dimensional transport in thin-layer and membrane cells, mixed conduction cases: Os(III)/Os(II) ClO4. J. Electroanal. Chem. 219:23–48; 1987.
Buck, R.P. Electron hopping in one dimension: Mixed conductor membranes. J. Phys. Chem. 92:4196–4200; 1988.
Buck, R.P. Steady state diffusion-migration potential differences in mixed conductor polymer films and thin layer cells. J. Electroanal. Chem. 271:1–14; 1989.
Buck, R.P. Impedances of membrane systems with metal and/or ionic contacts. Electrochim. Acta 35:1609–1617; 1990.
Buck, R.P.; Bronner, W.E. Prediction of salt effects on rates of single-ion crossings in ITIES experiments. J. Electroanal. Chem. 197:179–188, 1986.
Buck, R.P.; Mathis, D.E.; Rhodes, R.K. Impedance measurements on purified silver chloride crystals using ionic vs electronic contacts. J. Electroanal. Chem. 80:245–257; 1977.
Buck, R.P.; Stover, F.S.; Mathis, D.E. Site concentration determination in liquid ion exchange membranes: Theory and techniques. J. Electroanal. Chem. 82:345–360; 1977.
Buck, R.P.; Stover, F.S.; Mathis, D.E. Site concentration determination in liquid ion exchange membranes: Experimental. J. Electroanal. Chem. 100:63–70; 1979.
Buck, R.P.; Vanysck, P. Interfacial potential differences at mixed conductor interfaces: Nernst, Nerst-Donnan, Nernst Distribution and generalizations. J. Electroanal. Chem. 292:73–91; 1990.
Cole, K.S. Membranes, ions, and impulses. Berkeley, CA: University of California Press; 1972; pp. 167–203.
Cole, K.S. Membranes, ions, and impulses. Berkeley, CA: University of California Press; 1972; p. 303.
Franceschetti, D.R.; Macdonald, J.R. Electrode kinetics, equivalent circuits, and system characterization: Small-signal conditions. J. Electroanal. Chem. 82:271–301; 1977.
Franceschetti, D.R.; Macdonald, J.R. Diffusion of neutral and charged species under small-signal a.c. conditions. J. Electroanal. Chem. 101:307–316; 1979.
Franceschetti, D.R.; Macdonald, J.R. Small-signal response theory for electrochomic thin films. J. Electroanal. Chem. 120:1754–1756; 1982.
Franceschetti, D.R.; Macdonald, J.R.; Buck, R.P. Interpretation of finite-length-Warburg-type impedances in supported and unsupported electrochemical cells with kinetically reversible electrodes. J. Electrochem. Soc. 138:1368–1371; 1991.
Gratzl, M.; Pungor, E.; Buck, R.P. Impedance measurements for pressed-pellet electrode membranes based on silver iodide and silver iodide/silver sulfide with solution contacts. Anal. Chim. Acta 189: 217–228; 1986.
Hebb, M.B. Electrical conductivity of silver sulfide. J. Chem. Phys. 20:185–190; 1952.
Hills, G.J.; Jacobs, P.W.M.; Lakshminarayanaiah, N. Membrane potentials: Part I The theory of the e.m.f. of cells containing ion-exchanging membranes. Part II The measurement of the e.m.f. of cells containing the cation-exchange membrane, cross-linked polymethacrylic acid. Proc. Roy. Soc. A262: 246–279; 1961.
Ho, C.; Raistrick, I.D.; Huggins, R.A. Application of a-c techniques to the study of lithium diffusion in tungsten trioxide thin films. J. Electrochem. Soc. 127:343–350; 1980.
Horval, G.; Graf, E.; Toth, K.; Pungor, E.; Buck, R.P. Plasticized poly(vinyl chloride) properties and characteristics of valinomycin electrodes: High-frequency resistances and dielectric properties. Anal. Chem. 58:2735–2740; 1986.
Hunter, T.B.; Tyler, P.S.; Smyrl, W.H.; White, H.S. Impedance analysis of poly(vinylferrocene) films. J. Electrochem. Soc. 134:2198–2204; 1987.
Iglehart, M.L.; Buck, R.P. Ion transport properties of cyclic and acyclic neutral carrier-containing membranes. Talanta. 36:89–98; 1989.
Labes, R.; Lullies, H. Analyses der nerveneigenschaften durch wechselstrommessungen mit hilfe der membrankernleitertheorie. Arch. Ges. Physiol. 230:738–770; 1932.
Lindner, E.; Niegreisz, Zs.; Toth, K.; Pungor, E.; Berube, T.R.; Buck, R.P. Electrical and dynamic properties of non-plasticized potassium selective membranes. J. Electroanal. Chem. 259: 67–80; 1989.
Macdonald, J.R. Theory of space-charge polarization and electrode-discharge effects. J. Chem. Phys. 58:4982–5001; 1973.
Macdonald, J.R. Simplified impedance/frequency-response results for intrinsically conducting solids and liquids. J. Chem. Phys. 61:3977–3996; 1974.
Macdonald, J.R. Binary electrolyte small-signal frequency response. J. Electroanal. Chem. 53:1–55; 1974.
Macdonald, J.R. Some a.c. response results for solids with recombining space charge. J. Phys. C7:L327-L331; 1974.
Macdonald, J.R. Complex rate constant for an electrochemical system involving an adsorbed intermediate. J. Electroanal. Chem. 70:17–26; 1976.
Macdonald, J.R. Interpretation of ac impedance measurements in solids. In: Mahan, G.D.; Roth, W.L., eds. Superionic conductors. New York: Plenum Press; 1976: pp. 81–97.
Macdonald, J.R. Space charge polarisation. In: Kleitz, M.; Dupuy, J., eds. Electrode processes in solid state ionics. Dordrecht: Reidel; 1976: pp. 149–180.
Macdonald, J.R. Impedance spectroscopy—Emphasizing solid materials and systems. New York: Wiley-Interscience Pubs.; 1987.
Macdonald, J.R.; Franceschetti, D.R. Theory of small-signal a.c. response of solids and liquids with recombining mobile charges. J. Chem. Phys. 68:1614–1637; 1978.
Mathis, D.E.; Buck, R.P. Ion transport in free and supported nitrobenzene aliquat nitrate liquid membrane ion-selective electrodes I: Bulk electrical properties including ion association and dielectric constant. II: Interfacial kinetics and time-dependent phenomena. J. Membr. Sci. 4:379–414; 1979.
Nyikos, L.; Pajkossy, T. Diffusion to fractal surfaces parts I, II, and III. Electrochim. Acta 31:1347, 1986; 34:171–186; 1989.
Reid, J.D.; Vanysck, P.; Buck, R.P. Potential dependence of capacitance at a polarizable (blocked) liquid/liquid interface. J. Electroanal. Chem. 161:1–15; 1984.
Reid, J.D.; Vanysck, P.; Buck, R.P. Potential dependence of capacitance at a liquid/liquid interface, unblocked interface. J. Electroanal. Chem. 170:109–125; 1984.
Rhodes, R.K.; Buck, R.P. Impedance measurements using ionic contacts on purified and doped silver bromide crystals. J. Electroanal. Chem. 86:349–360; 1978.
Rubinstein, I.; Rishpon, J.; Gottesfeld, S. An a.c.-impedance study of electrochemical processes at Nafion-coated electrodes. J. Electrochem. Soc. 133:729–734; 1986.
Sandifer, J.R.; Buck, R.P. Impedance characteristics of ion selective glass electrodes. J. Electroanal. Chem. 56:385–398; 1974.
Stover, F.S.; Brumleve, T.R.; Buck, R.P. Comparison of time constants for liquid ion-exchange membrane electrode responses determined by an impedance method and an activity step method. Anal. Chim. Acta 109:259–278; 1979.
Stover, F.S.; Buck, R.P. Site concentration determination in liquid ion exchange membranes. J. Electroanal. Chem. 94:59–66; 1978.
Stover, F.S.; Buck, R.P. Electrical properties of mobile site ion exchange membranes with interfacial permselectivity breakdown: Non-zero current properties. J. Electroanal. Chem. 107:165–175; 1980.
Toth, K.; Graf, E.; Horvai, G.; Pungor, E.; Buck, R.P. Plasticized poly(vinylchloride) properties and characteristics of valinomyvcin electrodes. Part 2: Low-frequency, surface-rate and Warburg impedance characteristics. Anal. Chem. 58:2741–2744; 1986.
Vanysck, P. Electrochemistry on liquid/liquid interfaces: Lecture notes in chemistry No. 39. Berlin: Springer-Verlag; 1985.
Wagner, C. Galvanic cells with solid electrolytes involving ionic and electronic conduction. In: Proceedings of the 7th Meeting of the International Commission of Electrochemical Thermodynamics and Kinetics, London: Butterworths; 1957: pp. 361–377.
Wandlowski, T.; Marecek, V.; Samec, Z. Kinetic analysis of the picrate ion transfer across the interface between two immiscible electrolyte solutions from impedance measurements at the equilibrium potential. j. Electroanal. Chem. 242:291–302; 1988.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buck, R.P. Impedances of thin and layered systems: Cells with even or odd numbers of interfaces. Ann Biomed Eng 20, 363–383 (1992). https://doi.org/10.1007/BF02368537
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02368537