Summary
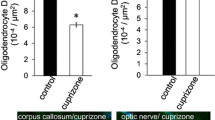
The glial response to Wallerian degeneration was studied in optic nerves following unilateral enucleation in immature rats, aged 21 days old (P21). The three-dimensional morphology of dye-filled glia was determined in intact nerves, at postenucleation day 21 and in normal nerves from untreated P21 rats, by correlating, laser scanning confocal microscopy andcamera lucida drawings of single cells. In normal and transected nerves, the majority of dye-filled cells comprized astrocytes (54% and 65%, respectively). In normal P21 nerves, the predominant astrocyte form had a complex stellate morphology and had a centrally-located cell body from which branching processes extended randomly. The other distinct forms were transverse and longitudinal astrocytes, which had a polarized process extension in a plane perpendicular or parallel to the long axis of the nerve, respectively. These forms were recognized in transected nerves also, but astrocytes in transected nerves had a simple morphology on the whole, and extended few, dense processes which branched infrequently. Quantitative analysis of astrocyte morphology confirmed that individual astrocytes underwent considerable remodelling in response to Wallerian degeneration. A prominent reaction was that astrocytes had withdrawn radial processes and extended a greater proportion of processes longitudinally, parallel to the long axis of the nerve and along the course of degenerated axons. A further, notable feature of transected nerves was the development of novel longitudinal forms and of hypertrophic astroglia. These results indicated that all astrocytes became reactive following enucleation and that glial scar formation was not the function of a single astrocyte subtype. Oligodendrocytes in transected nerves had lost their myelin sheaths and appeared as small cells with numerous bifurcating processes which extended radially, but a small number of oligodendrocytes were recognized which apparently supported myelin sheaths (9%, compared to 40% in normal nerves). In addition, there was a significant population of indeterminate cells in transected nerves (26%, compared to 6% in normal nerves) and, although some of these were identified as microglia/macrophages, it was concluded that many were likely to be dedifferentiated oligodendrocytes.
Similar content being viewed by others
References
Berry, M., Ibrahim, M., Carlile, J., Ruge, F., Duncan, A. &Butt, A. M. (1995) Axon-glial relationships in the anterior medullary velum of the adult rat.Journal of Neurocytology 24, 965–83.
Bignami, A. &Dahl, D. (1976) The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific (GFA) in mammalian and submammalian vertebrates.Neuropathology and Applied Neurobiology 2, 99–110.
Blaugrand, E., Duvdevani, R., Lavie, V., Solomon, A. &Schwarz, M. (1992) Disappearance of astrocytes and invasion of macrophages following crush injury of adult rodent optic nerves: implications for regeneration.Experimental Neurology 118, 105–15.
Butt, A. M. &Jenkins, H. G. (1994) Morphological changes in oligodendrocytes in the intact mouse optic nerve following intravitreal injection of tumour necrosis factor.Journal of Neuroimmunology 51, 27–33.
Butt, A. M. &Kirvell, S. (1996) Glial cells in transected optic nerves of immature rats. II. An immunohistochemical study.Journal of Neurocytology 25, 381–392.
Butt, A. M. &Ransom, B. R. (1989) Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of Lucifer, yellow and horseradish peroxidase.Glia 2, 470–5.
Butt, A. M. &Ransom, B. R. (1993) Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve.Journal of Comparative Neurology 338, 141–58.
Butt, A. M., Colquhoun, K. &Berry, M. (1994a) Confocal imaging of glial cells in the intact rat optic nerve.Glia 10, 315–22.
Butt, A. M., Colquhoun, K., Tutton, M. &Berry, M. (1994b) Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve.Journal of Neurocytology 23, 469–85.
Butt, A. M., Duncan, A. &Berry, M. (1994c) Astrocyte association with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve.Journal of neurocytology 23, 486–99.
Butt, A. M., Ibrahim, M., Ruge, F. &Berry, M. (1995) Biochemical subtypes of oligodendrocyte in the anterior medullary velum of the adult rat as revealed by the monoclonal antibody Rip.Glia 14, 185–7.
Calvo, J. L., Carbonell, A. L. &Boya, J. (1991) Coexpression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats.Brain Research 566, 333–6.
Carbonell, A. L., Boya, J., Calvo, J. L. &Marin, J. F. (1991) Ultrastructural study of the neuroglial and macrophagic reaction to Wallerian degeneration of the adult rat optic nerve.Histology and Pathology 6, 443–51.
Chan-Ling, T. &Stone J. (1991) Factors determining the morphology and distribution of astrocytes in the cat retina: a ‘contact-spacing’ model of astrocyte interaction.Journal of Comparative Neurology 303, 387–99.
Da Cunha, A., Jefferson, J. J., Tyor, W. R., Glass, J. D., Jannotta, F. S. &Vitkovic, L. (1993) Gliosis in human brain: relationship to size but not other properties of astrocytes.Brain Research 600, 161–5.
Dahl, D., Crosby, C. J. &Bignami, A. (1981) Filament proteins in rat optic nerves undergoing Wallerian degeneration: localization of vimentin, the fibroplastic 100-Å filament protein, in normal and reactive astrocytes.Experimental Neurology 73, 496–506.
Del Rio-Hortega, P. (1932) Microglia. InCytology and Cellular Pathology of the Nervous System, Vol. 2 (edited byPenfield, W.) pp. 481–534. New York: Hoeber.
Eddleston, M. &Mucke L. (1993) Molecular profile of reactive astrocytes — implications for their role in neurologic disease.Neuroscience 54, 15–36.
Franklin, R. J. &Blakemore, W. F. (1995) Glial-cell transplantation and plasticity in the O-2A lineageimplications for CNS repair.Trends in Neurosciences 18, 151–6.
Fulcrand, J. &Privat, A. (1977) Neuroglial reactions secondary to Wallerian degeneration in the optic nerve of the postnatal rat: ultrastructural and quantitative study.Journal of Comparative Neurology 176, 189–224.
Fulton, B. P., Burne, J. F. &Raff, M. C. (1992) Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake.Journal of Neuroscience 12, 4816–33.
Kidd, G. J., Hauer, P. E. &Trapp, B. D. (1990) Axons modulate myelin protein message RNA levels during central nervous system myelinationin vivo.Journal of Neuroscience Research 26, 409–18.
Lassman, H., Ammerer, H. P. &Kulnig, W. (1978) Ultrastructural sequence of myelin degradation. I. Wallerian degeneration in the rat optic nerve.Acta Neuropathologica 44, 91–102.
Lawson, L. J., Frost, L., Risbridger, J., Fearn, S. &Perry, V. H. (1994) Quantification of the mononuclear phagocyte response to Wallerian degeneration of the optic nerve.Journal of Neurocytology 23, 729–44.
Ludwin, S. K. (1990a) Phagocytosis in the rat optic nerve following Wallerian degeneration.Acta Neuropathologica 80, 266–73.
Ludwin, S. K. (1990b) Oligodendrocyte survival in Wallerian degeneration.Acta Neuropathologica 80, 184–91.
Mcphilemy, K., Griffiths, I. R., Mitchell, L. S. &Kennedy, P. G. E. (1991) Loss, of axonal contact causes down-regulation of the PLP gene in oligodendrocytes: evidence from partial lesions of the optic nerve.Neuropathology and Applied Neurobiology 17, 275–87.
Mathewson, A. J. &Berry, M. (1985) Observations on the astrocyte response to a cerebral stab wound in adult rats.Brain Research 327, 61–9.
Matthieu, J.-M., Compte, V., Tosic, M. &Honegger, P. (1992) Myelin gene expression during demyelination and remyelination in aggregating brain cell cultures.Journal of Neuroimmunology 40, 231–4.
Miller, R. H., David, S., Patel, R., Abney, E. R. &Raff, M. C. (1985) A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve:in vivo evidence for two distinct astrocyte lineages.Developmental Biology 111, 35–41.
Miller, R. H., Abney, E. R., David, S., Ffrenchconstant, C., Linsday, R., Patel, R., Stone, J. &Raff, M. C. (1986) Is reactive gliosis a property of a distinct subpopulation of astrocytes?Journal of Neuroscience 6, 22–9.
Miller, R. H., Fulton, B. P. &Raff, M. C. (1989) A novel type of glial cell associated with nodes of Ranvier in rat optic nerve.European Journal of Neuroscience 1, 172–80.
Miyake, T., Hattori, T., Fukuda, M., Kitamura, T. &Fujita, S. (1988). Quantitative studies on proliferative changes in reactive astrocytes in mouse cerebral cortex.Brain Research 451, 133–8.
Mori, S., &Leblond, C. P. (1969) Identification of microglia in light and electron microscopy.Journal of Comparative Neurology 135, 57–80.
Penfield, W. (1932)Cytology and Cellular Pathology of the Nervous System, vol. 2. New York: Hoeber.
Perry, V. H., Henderson, Z. &Linden, R. (1983) Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat.Journal of Comparative Neurology 219, 356–68.
Perry, V. H., Hume, D. A. &Gordon, S. (1985) Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain.Neuroscience 15, 313–26.
Perry, V. H., Brown, M. C. &Gordon, S. (1987) The macrophage response to central and peripheral nerve injury: a possible role for macrophages in regeneration.Journal of Experimental Medicie 165, 1218–23.
Privat, A., Valat, J. &Fulcrand, J. (1981) Proliferation of neuroglial cell lines in the degenerating optic nerve of young rats.Journal of Neuropathology and Experimental Neurology 40, 46–60.
Ramón-Moliner, E. (1958) A study of neuroglia. The problem of transitional forms.Journal of Comparative Neurology 110, 157–71.
Ransom, B. R., Butt, A. M. &Black, J. (1991) Ultrastructural identification of HRP-injected oligodendrocytes in the intact rat optic nerve.Glia 4, 37–45.
Reigner, J., Matthieu, J.-M., Kraus-Ruppert, R., Lassmann, H. &Poduslo, J. F. (1981) Myelin proteins, glycoproteins, and myelin-related enzymes in experimental demyelination of the rabbit optic nerve: sequence of events.Journal of Neurochemistry 36, 1986–95.
Schiffer, D., Giordana, M. T., Migheli, A., Giaccone, G., Pezzotta, S. &Mauro, A. (1986) Glial fibrillary acidic protein and vimentin in the experimental glial reaction of rat brain.Brain Research 374, 110–18.
Sefton, A. J. &Lam, K. (1984) Quantitative and morphological studies on developing optic axons in normal and enucleated albino rats.Experimental Brain Research 57, 107–17.
Skoff, R. P. (1975) The fine structure of pulse labelled (3H-thymidine cells) in degenerating rat optic nerve.Journal of Comparative Neurology 161, 595–612.
Stichel, C. C. &Müller, H.-W. (1994) Extensive and long-lasting changes of glial cells following transection of the postcomisural fornix in the adult rat.Glia 10, 89–100.
Stoll, G., Trapp, B. D. &Griffin, J. W. (1989) Macrophage function during Wallerian degeneration of rat optic nerve: clearance of degenerating myelin and la expression.Journal of Neuroscience 9, 2327–35.
Takamiya, Y., Kohsaka, S., Toya, S., Otani, M. &Tsukada, Y. (1988) Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats.Brain Research 38, 201–10.
Trimmer, P. A. &Wunderlich, R. E. (1990) Changes in astroglial scar formation in rat optic nerve as a function of development.Journal of Comparative Neurology 296, 359–78.
Vaughn, J. E. &Pease, D. C. (1970) Electron microscopic studies of Wallerian degeneration in rat optic nerves. II. Astrocytes, oligodendrocytes and adventitial cells.Journal of Comparative Neurology 140, 207–26.
Vaughn, J. E. &Skoff, R. P. (1972) Neuroglia in experimentally altered central nervous system. InThe Structure and Function of Nervous Tissue, vol. 5 (edited byBourne, G. H.), pp. 39–72. New York: Academic Press.
Vaughn, J. E., Hinds, P. L. &Skoff, R. P. (1970) Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia.Journal of Comparative Neurology 140, 175–206.
Wolswijk, G. (1994) G −D3 cells in the rat optic nerve are ramified microglia rather than O-2Aadult progenitor cells.Glia 10, 244–9.
Wolswijk, G. &Noble, M. (1995)in vitro studies of the development, maintenance and regeneration of the oligodendrocyte-type-2 astrocyte (O-2A) lineage in the adult central nervous system. InNeuroglia (edited byKettenman, H. &Ransom, B. R.) pp. 149–61. New York, Oxford: Oxford University Press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butt, A.M., Colquhoun, K. Glial cells in transected optic nerves of immature rats. I. An analysis of individual cells by intracellular dye-injection. J Neurocytol 25, 365–380 (1996). https://doi.org/10.1007/BF02284808
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02284808