Summary
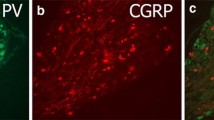
Using immunocytochemical and morphometric techniques, the localisation of three neuronal oligosaccharide antigens (two lactoseries and one globoseries oligosaccharide) were studied in the spinal cord and dorsal root ganglia of adult rats following unilateral crushing or transection of the sciatic nerve. The expression of CGRP and GAP43 was also studied for comparison. We found that following transection of the nerve the expression of lactoseries oligosaccharides and CGRP was permanently depressed, whilst that of the globoseries antigen (SSEA4) was unaffected. However following crush trauma and subsequent regeneration after 2 months, only the expression of one lactoseries antigen, LA4 remained significantly depressed. Our results suggest that different subsets of sensory neurons vary in the rate of reaction to injury and that one subset of neurons expressing a lactoseries oligosaccharide antigen is particularly susceptible to axotomy-induced changes. Furthermore neurons expressing the globoseries oligosaccharide antigen SSEA4 appear to be relatively unaffected by peripheral axotomy.
Similar content being viewed by others
References
Aldskogius, H. &Arvidsson, J. (1978) Nerve cell degeneration and death in the trigeminal ganglion of the adult rat following peripheral nerve transection.Journal of Neurocytology 7, 229–50.
Aldskogius, H. &Risling, M. (1981) The effect of sciatic neurectomy on neuronal number and size distribution in the L7 ganglion of kittens.Experimental Neurology 74, 597–604.
Aldskogius, H., Arvidsson, J. & Grant, G. (1992) Axotomy-induced changes in primary sensory neurons. InSensory Neurons (edited byScott, S. A.) pp. 363–83. Oxford University Press.
Alvarez, F. J., Rodrigo, J., Jessell, T. M., Dodd, J. &Priestley, J. V. (1989) Morphology and distribution of primary afferent fibres expressing α-galactose extended oligosaccharides in the spinal cord and brainstem of the rat. Light microscopy.Journal of Neurocytology 18, 611–29.
Alvarez, F. J., Morris, H. R. &Priestley, J. V. (1991) Sub-populations of smaller diameter trigeminal primary afferent neurons defined by expression of calcitonin gene-related peptide and the cell surface oligosaccharide recognised by monoclonal antibody LA4.Journal of Neurocytology 20, 716–31.
Arvidsson, J., Ygge, J. &Grant, G. (1986) Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat.Brain Research 373, 15–21.
Bondok, A. A. &Sansone, F. M. (1984) Retrograde and transganglionic degeneration of sensory neurons after a peripheral nerve lesion at birth.Experimental Neurology 86, 322–30.
Bridge, P. M., Ball, D. J., Mackinnon, S. E., Nakao, Y., Brandt, K., Hunter, D. A. &Hertl, C. (1994) Nerve crush injuries — a model for axonotmesis.Experimental Neurology 127, 284–90.
Burry, R. W., Lah, J. J. &Hayes, D. M. (1992) GAP-43 distribution is correlated with development of growth cones and presynaptic terminals.Journal of Neurocytology 21, 413–25.
Cameron, A. A., Cliffer, K. D., Dougherty, P. M., Willis, W. D. &Carlton, S. M. (1991) Changes in lectin, GAP43, and neuropeptide staining in the rat superficial dorsal horn following experimental peripheral neuropathy.Neuroscience Letters 131, 249–52.
Carr, P. A., Yamamoto, T. &Nagy, J. I. (1990) Calcitonin gene-related peptide in primary afferent neurons of rat: co-existence with fluoride-resistant acid phosphatase and depletion by neonatal capsaicin.Neuroscience 36, 751–60.
Cavanaugh, M. V. (1951) Quantitative effects of the peripheral innervation area on nerves and spinal ganglion cells.Journal of Comparative Neurology 94, 181–219.
Chou, D. K. H., Dodd J., Jessell, T. M., Costello C. E. &Jungawala, F. B. (1989) Identification of α-galactose (α-fucose)-asialo-GM1 glycolipid expressed by subsets of rat dorsal root ganglion neurons.Journal of Biological Chemistry 264, 3409–15.
Curtis, R., Green, D., Lindsay, R. M. &Wilkin, G. P. (1993) Up-regulation of GAP43 and growth of axons in rat spinal cord after compression injury.Journal of Neurocytology 22, 51–64.
De Graan, P. N. E., Van Hooff, C. O. M., Tilly, B. C., Oestreicher, A. B., Schotman, P. &Gispen, W. H. (1985) Phosphoprotein B-50 in nerve growth cones from fetal rat brain.Neuroscience Letters 61, 235–41.
Dodd, J., Solter, D. &Jessell, T. M. (1984) Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurons.Nature 311, 469–72.
Fitzgerald, M., Reynolds, M. L. &Benowitz, L. I. (1991) GAP-43 expression in the developing rat lumbar spinal cord.Neuroscience 41, 187–99.
Gibson, S. J., Polak, J. M., Bloom, S. R., Sabate, I. M., Mulderry, P. M., Ghatei, M. A., McGregor, G. P., Morrison, J. F. B., Kelley, J. S., Evans, R. M. &Rosenfeld, M. G. (1984) Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species.Journal of Neuroscience 4, 3101–11.
Gorgels, T. G. M. F., Van Lookeren Campagne, M., Oestreicher, A. B., Gribnau, A. A. M. &Gispen, W. H. (1989) B-50/GAP43 is localised at the cytoplasmic side of the plasma membrane in developing and adult rat pyramidal tract.Journal of Neuroscience 9, 3861–9.
Gundersen, H. J. G., Bagger, P., Bendtsen, T. F., Evans, S. M., Korbo, L., Marcussen, N., Møller, A., Nielsen, K., Nyengaard, J. R., Pakkenberg, B., Sørenson, F. B., Vesterby, A. &West, M. J. (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis.Acta Pathologica, Microbiologica et Immunologica Scandinavica 96, 857–81.
Himes, B. T. &Tessler, A. (1989) Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats.Journal of Comparative Neurology 284, 215–30.
Hökfelt, T., Elde, R., Johansson, O., Luft, R., Nilsson, G. &Arimura, A. (1976) Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat.Neuroscience 1, 131–6.
Inaishi, Y., Kashihara, Y., Sakaguchi, M., Nawa, H. &Kuno, M. (1992) Cooperative regulation of calcitonin gene-related peptide levels in rat sensory neurons via their central and peripheral processes.Journal of Neuroscience 12, 518–24.
Jeftinija, S. &Jeftinija, K. (1990) Calcitonin gene-related peptide immunoreactivity in neuronal perikarya in dorsal root.Brain Research 519, 324–8.
Jessell, T. M. &Dodd, J. (1985) Structure and expression of differentiation antigens on functional subclasses of primary sensory neurons.Philosophical Transactions of the Royal Society of London, Series B 308, 271–81.
Jessell, T. M., Tsunoo, A., Kanazawa, I. &Otsuka, M. (1979) Substance P: depletion in the dorsal horn of rat spinal cord after section of the peripheral processes of primary sensory neurons.Brain Research 168, 247–59.
Kalil, K. &Skene, J. H. P. (1986) Elevated synthesis of an axonally transported protein correlates with axon outgrowth in normal and injured pyramidal tracts.Journal of Neuroscience 6, 2563–70.
Kar, S., Gibson, S. J., Scaravilli, F., Jacobs, J. M., Aber, V. R. &Polak, J. M. (1989) Reduced numbers of calcitonin gene-related peptide (CGRP) and tachykinin-immunoreactive sensory neurones associated with greater enkephalin immunoreactivity in the dorsal horn of a mutant rat with hereditary sensory neuropathy.Cell and Tissue Research 255, 451–66.
Knyihár, E. (1971) Fluoride-resistant acid phosphatase system of nociceptive dorsal root afferents.Experentia 27, 1205–7.
Knyihár, E. &Csillik, B. (1976) Effect of peripheral axotomy on the fine structure and histochemistry of the Rolando substance: degenerative atrophy of central processes of pseudounipolar cells.Experimental Brain Research 26, 73–87.
Knyihár-Csillik, E., Török, A. &Csillik, B. (1990) Primary afferent origin of substance P-containing axons in the superficial dorsal horn of the rat spinal cord: depletion, regeneration and replenishment of presumed nociceptive central terminals.Journal of Comparative Neurology 297, 594–612.
Lawson, S. N. (1992) Morphological and biochemical cell types of sensory neurons. InSensory Neurons (edited byScott, S. A.) pp 27–59. Oxford University Press.
Lawson, S. N., Harper, A. A., Harper, E. I., Garson, J. A. &Anderton, B. H. (1984) A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones.Journal of Comparative Neurology 228, 263–72.
Lieberman, A. R. (1976) Sensory ganglia. InThe Peripheral Nerve (edited byLandon, D. N.), pp. 188–278. London: Chapman and Hall.
Matsuda, Y., Yoshida, S. &Yonezawa, T. (1978) Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells culturedin vitro.Brain Research 154, 69–82.
Meller, K. (1989) Chromatolysis of dorsal root ganglion cells studied by cryofixation.Cell and Tissue Research 256, 283–92.
Nagy, J. I. &Hunt, S. P. (1982) Fluoride-resistant acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin.Neuroscience 7, 89–97.
Pearson, R. C. A., Taylor, N. &Snyder, S. H. (1988) Tubulin messenger RNA:in situ hybridization reveals bilateral increases in hypoglossal and facial nuclei following nerve transection.Brain Research 463, 245–9.
Peyronnard, J-M., Charron, L., Messier, J-P., Lavoie, J., Leger, C. &Faraco-Cantin, F. (1989) Changes in lectin binding of dorsal lumbar root ganglia neurons and peripheral axons after sciatic and spinal nerve injury in the rat.Cell and Tissue Research 257, 379–88.
Plenderleith, M. B. &Snow, P. J. (1990) The effect of peripheral nerve section on lectin binding in the superficial dorsal horn of the rat spinal cord.Brain Research 507, 146–50.
Ranson, S. W. (1909) Alterations in the spinal ganglion cells following axotomy.Journal of Comparative Neurology 19, 125–53.
Rexed, B. (1952) The cytoarchitectonic organisation of the spinal cord in the cat.Journal of Comparative Neurology 96, 415–95.
Rich, K. M., Disch, S. P. &Eichler, M. E. (1989) The influence of regeneration and nerve growth factor on the neuronal cell body reaction to injury.Journal of Neurocytology 18, 569–76.
Risling, M., Aldskogius, H., Hildebrand, C. &Remahl, S. (1983) Effects of sciatic nerve resection on L7 spinal root and dorsal root ganglia in adult cats.Experimental Neurology 72, 568–80.
Schmalbruch, H. (1987) Loss of sensory neurons after sciatic nerve section in the rat.Anatomical Record 219, 323–9.
Schreyer, D. J. &Skene, J. H. P. (1991) Fate of GAP43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential.Journal of Neuroscience 11, 3738–51.
Shortland, P. &Woolf, C. J. (1993) Chronic peripheral nerve section results in a rearrangement of the central axonal arborizations of axotomised A beta primary afferent neurons in the rat spinal cord.Journal of Comparative Neurology 330, 65–82.
Silverman, J. D. &Kruger, L. (1990) Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers.Journal of Neurocytology 19, 789–801.
Skene, J. H. P. &Willard, M. (1981) Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems.Journal of Cell Biology 89, 96–103.
Sommervaille, T., Reynolds, M. L. &Woolf, C. J. (1991) Time dependent differences in the increase in GAP43 expression in dorsal root ganglion cells after peripheral axotomy.Neuroscience 45, 213–20.
Tajti, J., Fischer, J., Knyihár-Csillik, E. &Csillik, B. (1988) Transganglionic regulation and fine structural localisation of lectin-reactive carbohydrate epitopes in primary sensory neurons of the rat.Histochemistry 88, 213–18.
Verge, V. M. K., Richardson, P. M., Benoit, R. &Riopelle, R. J. (1989) Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor.Journal of Neurocytology 18, 583–91.
Villar, M. J., Cortés, R., Theodorsson, E., Wiesenfeld-Hallin, Z., Schalling, M., Fahrenkrug, J., Emson, P. C. &Hökfelt, T. (1989) Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin.Neuroscience 33, 587–604.
Wang, H., Rivero-Melián, C., Robertson, B. &Grant, G. (1994) Transganglionic transport and binding of the isolectin B4 fromGriffonia simplicifolia I in rat primary sensory neurons.Neuroscience 62, 539–51.
White, F. A., Bennett-Clarke, C. A., MacDonald, G. J., Enfiejian, H. L., Chiaia, N. L. &Rhoades, R. W. (1990) Neonatal infraorbital nerve transection in the rat: comparison of effects on substance P immunoreactive primary afferents and those recognised by the lectinBandierea simplicifolia-I.Journal of Comparative Neurology 300, 249–62.
Williams, R. W. &Rakic, P. (1988) Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material.Journal of Comparative Neurology 278, 344–52.
Wong, J. &Oblinger, M. M. (1990) A comparison of peripheral and central axotomy effects on neurofilament and tubulin gene expression in rat dorsal root ganglion neurons.Journal of Neuroscience 10, 2215–22.
Woolf, C. J., Reynolds, M. L., Molander, C., O'Brien, C., Lindsay, R. M. &Benowitz, L. I. (1990) The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury.Neuroscience 34, 465–78.
Woolf, C. J., Shortland, P. &Coggeshall, R. E. (1992) Peripheral nerve injury triggers central sprouting of myelinated afferents.Nature 355, 75–7.
Ygge, J. (1989a) Neuronal loss in dorsal root ganglia after proximal compared to distal sciatic nerve resection: a quantitative study in the rat.Brain Research 478, 193–5.
Ygge, J. (1989b) Central projections of the rat radial nerve investigated with transganglionic degeneration and transganglionic transport of horseradish peroxidase.Journal of Comparative Neurology 279, 199–211.
Zhang, X., Ju, G., Elde, R. &Hökfelt, T. (1993) Effect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galanin.Journal of Neurocytology 22, 342–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Groves, M.J., Yin, W.N., Ciardi, A. et al. Sciatic nerve injury in the adult rat: comparison of effects on oligosaccharide, CGRP and GAP43 immunoreactivity in primary afferents following two types of trauma. J Neurocytol 25, 219–231 (1996). https://doi.org/10.1007/BF02284798
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02284798