Abstract
The physiology of spore-negative and spore-positive root nodules was investigated inMyrica gale L. grown in water culture in a growth chamber. Spore(−) nodules were induced withFrankia cultures and spore(+) nodules with crushed nodules. Gas exchange was measured in a flow-through system.
The time course of acetylene reduction following addition of acetylene was essentially the same in both spore(−) and spore(+) nodules with a stable maximum between 2 and 4 minutes followed by a steep decline to a minimum (37% of the maximum) between 9 and 30 minutes depending on the plant. The minimum was followed by a partial recovery. Nodule CO2 evolution showed a similar pattern but the minimum rate (83% of the maximum) was not nearly as low.
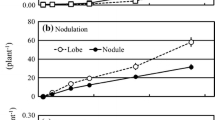
Plants nodulated with one spore(−) and one spore(+) strain were compared at 6, 8 and 10 weeks after inoculation. At 6 weeks the spore(−) plants had 52% greater specific nitrogenase activity and 46% more biomass than the spore(+) plants. At 8 and 10 weeks, however, the differences between plants with spore(−) and spore(+) nodules became smaller.
Plants nodulated with 4 spore(−) and 5 spore(+) strains were compared at 8 weeks after inoculation. Collectively the spore(−) plants exhibited a 32% greater specific nitrogenase activity, a 15% lower energy cost of nitrogenase activity (CO2/C2H4), and invested 31% less biomass in nodules than the spore(+) plants. The spore(−) plants also produced 16% more biomass indicating that spore(−) strains are generally more desirable than spore(+) strains. However, two spore(+) strains were as effective as the spore(−) strains.
Similar content being viewed by others
References
Burggraaf A J P, Quispel A, Tak T and Valstar J 1981 Methods of isolation and cultivation ofFrankia species from actinorhizas. Plant and Soil 61, 157–168.
Daniere C, Capellano A and Moiroud A 1986 Dynamique de l'azote dans un peuplement naturel d'Alnus incana (L.) Moench. Acta Oecol.-Oecol. Plant 7, 165–175.
Hall R B, McNabb H S Jr., Maynard C A and Green T L 1979 Toward development of optimalAlnus glutinosa symbioses. Bot. Gaz 140 (Suppl.), S120–126.
Hoagland D R and Arnon D I 1950 The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347 (rev. ed.).
Holman R M and Schwintzer C R 1987 Distribution of sporepositive and spore-negative nodules ofAlnus incana ssp.rugosa in Maine, USA. Plant and Soil 104, 103–111.
Hooker J E and Wheeler C T 1987 The effectivity ofFrankia for nodulation and nitrogen fixation inAlnus rubra andA. glutinosa. Physiol. Plant. 70, 333–341.
Huss-Danell K and Alqvist A-S 1984 Nitrogenase activity in root nodule homogenates ofAlnus incana. Plant and Soil 78, 159–170.
Kashanski C R and Schwintzer C R 1987 Distribution of sporepositive and spore-negative nodules ofMyrica gale in Maine, USA. Plant and Soil 104, 113–120.
Lalonde M, Simon L, Bousquet J and Seguin A 1988 Advances in the taxonomy ofFrankia: Recognition of speciesalni andelaeagni and novel subspeciesPOMMERII andVANDIJKII.In Proceedings—VII International Congress on Nitrogen Fixation. Gustav Fischer Verlag, Stuttgart.
Lechevalier M 1985 Catalog ofFrankia strains. The Actinomycetes 19, 131–162.
Lechevalier M P, Baker D and Horriere F 1983 Physiology, chemistry, serology and infectivity of twoFrankia isolates fromAlnus incana ssp.rugosa. Can. J. Bot. 61, 2826–2833.
Leopold A C and Kriedemann P E 1975 Plant Growth and Development, 2nd ed. McGraw-Hill Book Co., New York, 545 p.
Malcolm D C, Hooker J E and Wheeler C T 1985Frankia symbiosis as a source of nitrogen in forestry: a case study of symbiotic nitrogen-fixation in a mixed Alnus-Picea plantation in Scotland. Proc. R. Soc. Edinburgh 85B, 263–282.
Minchin F R, Sheehy J E and Witty J F 1986 Further errors in the acetylene reduction assay: effects of plant disturbance. J. Exp. Bot. 37, 1581–1591.
Minchin F R, Witty J F, Sheehy J E and Muller M 1983 A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J. Expt. Bot. 34, 641–649.
Monz C A 1988 Consequences of spore(−) and spore(+) nodules ofMyrica gale for nodule physiology and plant growth. MS thesis, University of Maine, Orono, 65 p.
Nesme X, Normand P, Tremblay F M and Lalonde M 1985 Nodulation speed ofFrankia sp. onAlnus glutinosa, Alnus crispa, andMyrica gale. Can. J. Bot. 63, 1292–1295.
Noggle G R and Fritz G J 1983 Introductory Plant Physiology. 2nd edition. Prentice Hall, Inc., Englewood Cliffs, New Jersey. 627 p.
Normand P and Lalonde M 1982 Evaluation ofFrankia strains isolated from provenances of twoAlnus species. Can. J. Microbiol. 28, 1133–1142.
Schwintzer C R, Berry A M and Disney L D 1982 Seasonal patterns of root nodule growth, endophyte morphology, nitrogenase activity, and shoot development inMyrica gale. Can. J. Bot. 60, 746–757.
Schwintzer C R and Tjepkema J D 1983 Seasonal pattern of energy use, respiration, and nitrogenase activity in root nodules ofMyrica gale. Can. J. Bot. 61, 2937–2942.
Schubert K R and Evans H J 1976 Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc. Natl. Acad. Sci. USA 73, 1207–1211.
Sellstedt A 1988 Nitrogenase activity, hydrogen evolution and biomass production in different Casuarina symbioses. Plant and Soil 105, 33–40.
Shipton W A and Burggraaf A J P 1982 A comparison of the requirements for various carbon and nitrogen sources and vitamins in someFrankia isolates. Plant and Soil 69, 149–161.
Silvester W B, Whitbeck J, Silvester J K and Torrey J G 1988 Growth, nodule morphology and nitrogenase activity ofMyrica gale with roots grown at various oxygen levels. Can. J. Bot., 66, 1762–1771.
Simon L, Stein A, Cote S and Lalonde M 1985 Performance ofin vitro propagatedAlnus glutinosa (L.) Gaertn. clones inoculated withFrankiae. Plant Soil 87, 125–133.
Tjepkema J D 1985 Utilization of photosynthate for nitrogen fixation in seedlings ofMyrica gale andAlnus rubra.In Nitrogen Fixation and CO2 Metabolism. pp 183–192. Elsevier Science Publishing, New York.
Tjepkema J D, Omerod W and Torrey J G 1981 Factors affecting vesicle formation and acetylene reduction (nitrogenase activity) inFrankia sp. Cpl1. Plant and Soil 27, 815–823.
Tjepkema J D, Schwintzer C R and Benson D R 1986 Physiology of actinorhizal nodules. Annu. Rev. Plant Physiol. 37, 209–232.
Tjepkema J D, Schwintzer C R and Monz C A 1988 Time course of acetylene reduction in nodules of five actinorhizal genera. Plant Physiol. 86, 581–583.
Torrey J G 1987 Endopyte sporulation in root nodules of actinorhizal plants. Physiol. Plant. 70, 279–288.
VandenBosch K A and Torrey J G 1984 Consequences of sporangial development for nodule function in root nodules ofComptonia peregrina andMyrica gale. Plant Physiol. 76, 556–560.
VandenBosch K A and Torrey J G 1985 Development of endophyticFrankia sporangia in field- and laboratory-grown nodules ofComptonia peregrina andMyrica gale. Am. J. Bot. 72, 99–108.
van Dijk C 1978 Spore formation and endophyte diversity in root nodules ofAlnus glutinosa (L.) Vill. New Phytol. 81, 601–615.
Wheeler C T, Hooker J E, Crowe A and Berrie A M M 1986 The improvement and utilization in forestry of nitrogen fixation by actinorhizal plants with special reference toAlnus in Scotland. Plant and Soil 90, 393–406.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Monz, C.A., Schwintzer, C.R. The physiology of spore-negative and spore-positive nodules ofMyrica gale . Plant Soil 118, 75–87 (1989). https://doi.org/10.1007/BF02232792
Issue Date:
DOI: https://doi.org/10.1007/BF02232792