Summary
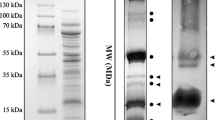
Antibodies to individual chloroplast ribosomal (r-)proteins ofChlamydomonas reinhardtii synthesized in either the chloroplast or the cytoplasm were used to examine the relatedness ofChlamydomonas r-proteins to r-proteins from the spinach (Spinacia oleracea) chloroplast,Escherichia coli, and the cyanobacteriumAnabaena 7120. In addition,35S-labeled chloroplast r-proteins from large and small subunits ofC. reinhardtii were coelectrophoresed on 2-D gels with unlabeled r-proteins from similar subunits of spinach chloroplasts,E. coli, andAnabaena to compare their size and net charge. Comigrating protein pairs were not always immunologically related, whereas immunologically related r-protein pairs often did not comigrate but differed only slightly in charge and molecular weight. In constrast, when35S-labeled chloroplast r-proteins from large and small subunits of a closely related speciesC. smithii were coelectrophoresed with unlabeledC. reinhardtii chloroplast r-proteins, only one pair of proteins from each subunit showed a net displacement in mobility.
Analysis of immunoblots of one-dimensional SDS and two-dimensional urea/SDS gels of large and small subunit r-proteins from these species revealed more antigenic conservation among the four species of large subunit r-proteins than small subunit r-proteins.Anabaena r-proteins showed the greatest immunological similarity toC. reinhardtii chloroplast r-proteins. In general, antisera made against chloroplast-synthesized r-proteins inC. reinhardtii showed much higher levels of cross-reactivity with r-proteins fromAnabaena, spinach, andE. coli than did antisera to cytoplasmically synthesized r-proteins. All spinach r-proteins that cross-reacted with antisera to chloroplast-synthesized r-proteins ofC. reinhardtii are known to be made in the chloroplast (Dorne et al. 1984b). FourE. coli r-proteins encoded by the S10 operon (L2, S3, L16, and L23) were found to be conserved immunologically among the four species. Two of the large subunit r-proteins, L2 and L16, are essential for peptidyltransferase activity. The third (L23) and two otherE. coli large subunit r-proteins (L5 and L27) that have immunological equivalents among the four species are functionally related to but not essential for peptidyltransferase activity.
Similar content being viewed by others
References
Auron PE, Fahnestock SR (1981) Functional organization of the large ribosomal subunit ofBacillus stearothermophilus. J Biol Chem 256:10105–10110
Bartsch M (1985) Correlation of chloroplast and bacterial ribosomal proteins by cross-reactions of antibodies specific to purifiedEscherichia coli ribosomal proteins. J Biol Chem 260:237–241
Bartsch M, Kimura M, Subramanian AR (1982) Purification, primary structure, and homology relationships of a chloroplast ribosomal protein. Proc Natl Acad Sci USA 79:6871–6875
Bonner WM, Laskey RA (1974) A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 46:83–88
Bourque DP, Capel MS (1982) Isolation and purification of tobacco chloroplast ribosomes. In: Edelman M, Hallick RB, Chua NH (eds) Methods in chloroplast molecular biology. Elsevier Biomedical Press, Amsterdam, pp 617–628
Boynton JE, Gillham NW, Lambowitz AM (1980) Biogenesis of chloroplast and mitochondrial ribosomes. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 903–950
Cooperman BS (1980) Functional sites on theE. coli ribosome as defined by affinity labeling. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 531–554
Dohme F, Nierhaus KH (1976) Role of 5S RNA in assembly and function of the 50S subunit fromEscherichia coli. Proc Natl Acad Sci USA 73:2221–2225
Dorne AM, Eneas-Filho J, Heizmann P, Mache R (1984a) Comparison of ribosomal proteins of chloroplast from spinach and ofE. coli. Mol Gen Genet 193:129–134
Dorne AM, Lescure AM, Mache R (1984b) Site of synthesis of spinach chloroplast ribosomal proteins and formation of incomplete ribosomal particles in isolated chloroplasts. Plant Mol Biol 3:83–90
Duggleby RG, Kaplan H, Visentin LP (1975) Carboxyl-terminal sequences of procaryotic ribosomal proteins fromEscherichia coli Bacillus stearothermophilus, andHalobacterium cutirubrum. Can J Biochem 53:827–833
Eneas-Filho J, Hartley MR, Mache R (1981) Pea chloroplast ribosomal proteins: characterization and site of synthesis. Mol Gen Genet 184:484–488
Erdmann VA, Fahnestock S, Higo K, Nomura M (1971) Role of 5S RNA in the functions of 50S ribosomal subunits. Proc Natl Acad Sci USA 68:2932–2936
Fahnestock S, Erdmann V, Nomura M (1973) Reconstitution of 50S ribosomal subunits from protein-free ribonucleic acid. Biochemistry 12:220–224
Fahnestock SR, Strycharz WA, Marquis DM (1981) Immunological evidence of homologies among 50S ribosomal proteins ofBacillus stearothermophilus andEscherichia coli. J Biol Chem 256:10111–10116
Fleming GH, Boynton JE, Gillham NW (1987) Cytoplasmic ribosomal proteins fromChlamydomonas reinhardtii: characterization and immunological comparisons. Mol Gen Genet 206:226–237
Hampl H, Schulze H, Nierhaus KH (1981) Ribosomal components fromEscherichia coli 50S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem 256:2284–2288
Hancock K, Tsang VCW (1983) India ink staining of proteins on nitrocellulose paper. Anal Biochem 133:157–162
Harris EH, Boynton JE, Gillham NW (1987) Chloroplast genome ofChlamydomonas reinhardtii. In: O'Brien SJ (ed) Genetic maps 1987. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY, pp 266–275
Horne JR, Erdmann VA (1972) Isolation and characterization of 5S RNA-protein complexes fromBacillus stearothermophilus andEscherichia coli ribosomes. Mol Gen Genet 119:337–344
Isono K (1980) Genetics of ribosomal proteins and their modifying and processing enzymes inEscherichia coli. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 641–669
Kimura J, Kimura M (1987) The complete amino acid sequences of the 5S rRNA binding proteins L5 and L18 from the moderate thermophileBacillus stearothermophilus ribosome. FEBS Lett 210:85–90
Kimura M, Chow CK (1984) The complete amino acid sequences of ribosomal proteins L17, L27 and S9 fromBacillus stearothermophilus. Eur J Biochem 139:225–234
Kimura M, Kimura J, Ashman K (1985a) The complete primary structure of ribosomal proteins L1, L14, L15, L23, L24, and L29 fromBacillus stearothermophilus. Eur J Biochem 150:491–497
Kimura M, Kimura J, Watanabe K (1985b) The primary structure of ribosomal protein L2 fromBacillus stearothermophilus. Eur J Biochem 153:289–297
Kratz WA, Myers J (1955) Nutrition and growth of several blue-green algae. Am J Bot 42:282–287
Kyriakopoulos A, Subramanian AR (1977) Positions of individual ribosomal proteins after two-dimensional electrophoresis by a sensitive procedure. Biochim Biophys Acta 474:308–311
Lennox ES (1955) Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206
Liu XQ, Gillham NW, Boynton JE (1988) Chloroplast ribosomal protein L-18 inChlamydomonas reinhardtii is processed during ribosome assembly. Mol Gen Genet 214:588–591
Lou JK, Wu M, Chang CH, Cuticchia AJ (1987) Localization of a r-protein gene within the chloroplast DNA replication origin ofChlamydomonas. Curr Genet 11:537–541
Lührmann R, Bald R, Stöffler-Meilicke M, Stöffler G (1981) Localization of the puromycin binding site on the large ribosomal subunit ofEscherichia coli by immunoelectron microscopy. Proc Natl Acad Sci USA 78:7276–7280
Mache R, Dorne AM, Batlle RM (1980) Characterization of spinach plastid ribosomal proteins by two dimensional gel electrophoresis. Mol Gen Genet 177:333–338
Mache R, Audren H, Bisanz-Seyer C, Dorne AM, Massenet O, Rozier C, Thomas F (1987) Biosynthesis of chloroplast ribosomal components. In: Biggens J (ed) Progress in photosynthesis research, vol IV. Martinus Nijhoff Publishers, Dordrecht, pp 547–551
Madjar JJ, Michel S, Cozzone AJ, Reboud JP (1979) A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application toEscherichia coli ribosomal proteins. Anal Biochem 92:174–182
Marquis DM, Fahnestock SR (1980) Stoichiometry and structure of a complex of acidic ribosomal protiens. J Mol Biol 142:161–179
Matheson AT, Möller W, Amons R, Yaguchi M (1980) Comparative studies on the structure of ribosomal proteins, with emphasis on the alanine-rich, acidic ribosomal ‘A’ protein. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 297–332
Möller W (1974) The ribosomal components involved in EFG-and EF-Tu-dependent GTP hydrolysis. In: Nomura M, Tissières A, Lengyel P (eds) Ribosomes. Cold Spring Harbor Laboratory, Cold Spring Harbor NY, pp 711–731
Myers AM, Harris EH, Gillham NW, Boynton JE (1984) Mutations in a nuclear gene ofChlamydomonas cause the loss of two chloroplast ribosomal proteins, one synthesized in the chloroplast and the other in the cytoplasm. Cur Genet 8:369–378
Nierhaus KH (1980) Analysis of the assembly and function of the 50S subunit fromEscherichia coli ribosomes by reconstitution. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 267–294
Nierhaus KH, Dohme F (1979) Total reconstitution of 50S subunits fromEscherichia coli ribosomes. Methods Enzymol 59:443–449
Nomura M, Post LE (1980) Organization of ribosomal genes and regulation of their expression inEscherichia coli. In: Chambliss G, Crayen GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press, Baltimore, pp 671–691
Ofengand J, Ciesiolka J, Denman R, Nurse K (1985) Structural and functional interactions of the tRNA-ribosome complex. In: Hardesty B, Kramer G (eds) Structure, function and genetics of ribosomes. Springer-Verlag, New York, pp 473–494
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwortMarchantia polymorpha chloroplast DNA. Nature 322:572–574
Olson HM, Grant PG, Cooperman BS, Glitz DG (1982) Immunoelectron microscopic localization of puromycin binding on the large subunit of theEscherichia coli ribosome. J Biol Chem 257:2649–2656
Schmidt G, Strobel O, Stöffler-Meilicke M, Stöffler G, Böck A (1984) A ribosomal protein that is immunologically conserved in archaebacteria, eubacteria and eukaryotes. FEBS Lett 177:189–194
Schmidt RJ, Richardson CB, Gillham NW, Boynton JE (1983) Sites of synthesis of chloroplast ribosomal proteins inChlamydomonas. J Cell Biol 96:1451–1463
Schmidt RJ, Myers AM, Gillham NW, Boynton JE (1984) Immunological similarities between specific chloroplast ribosomal proteins fromChlamydomonas reinhardtii and ribosomal proteins fromEscherichia coli. Mol Biol Evol 1:317–324
Schmidt RJ, Hosler JP, Gillham NW, Boynton JE (1985) Biogenesis and evolution of chloroplast ribosomes: cooperation of nuclear and chloroplast genes. In: Steinback K, Bonitz Z, Arntzen C, Bogorad L (eds) Molecular biology of the photosynthetic apparatus. Cold Spring Harbor Laboratory, Cold Spring Harbor NY, pp 417–427
Schulze H, Nierhaus KH (1982) Minimal set of ribosomal components for reconstitution of the peptidyltransferase activity. EMBO J 1:609–613
Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, Ohto C, Torazawa K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M (1986) The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5:2043–2049
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid inChlamydomonas reinhardtii. Proc Natl Acad Sci USA 46:83–89
Tanaka M, Wakasugi T, Sugita M, Shinozaki K, Sugiura M (1986) Genes for the eight ribosomal proteins are clustered on the chloroplast genome of tobacco (Nicotiana tabacum): similarity to the S10 andspc operons ofEscherichia coli. Proc Natl Acad Sci USA 83:6030–6034
Visentin LP, Matheson AT, Yaguchi M (1974) Homologies in procaryotic ribosomal proteins: alanine rich acidic proteins associated with polypeptide translation. FEBS Lett 41:310–314
Vogel DW, Hartmann RK, Bartsch M, Subramanian AR, Kleinow W, O'Brien TW, Pieler T, Erdmann VA (1984) Reconstitution of 50S ribosomal subunits fromBacillus stearothermophilus with 5S RNA from spinach chloroplasts and low-Mr RNA from mitochondria ofLocusta migratoria and bovine liver. FEBS Lett 169:67–72
Weissbach H (1980) Soluble factors in protein synthesis. In: Chambliss G, Craven GR, Davies J, Davis K, Kahan L, Nomura M (eds) Ribosomes: structure, function and genetics. University Park Press. Baltimore, pp 377–411
Wittmann HG (1982) Components of bacterial ribosomes. Annu Rev Biochem 51:155–183
Wittmann-Liebold B (1985) Ribosomal proteins: their structure and evolution. In: Hardesty B, Kramer G (eds) Structure, function and genetics of ribosomes. Springer-Verlag, New York, pp 326–361
Yu RST, Wittmann HG (1973) The sequence of steps in the attachment of 5S RNA to cores ofEscherichia coli ribosomes. Biochim Biophys Acta 324:375–385
Zimmerman RA (1979) Protein-RNA interactions in the bacterial ribosome. Methods Enzymol 59:551–583
Zurawski G, Bottomley W, Whitfeld PR (1984) Junctions of the large single copy region and the inverted repeats inSpinacia oleracea andNicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res 12:6547–6558
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Randolph-Anderson, B.L., Gillham, N.W. & Boynton, J.E. Electrophoretic and immunological comparisons of chloroplast and prokaryotic ribosomal proteins reveal that certain families of large subunit proteins are evolutionarily conserved. J Mol Evol 29, 68–88 (1989). https://doi.org/10.1007/BF02106183
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02106183