Summary
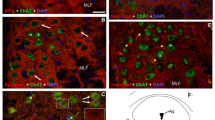
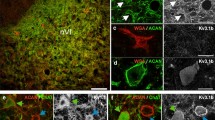
Monoclonal antibodies (McAB) specific for fast (C14) and slow (S58) myosin, and a myosin antigenically similar to neonatal/embryonic myosin in mammals (ALD180), were used to characterize the myosin distribution in orbital layer fibres of rat extraocular muscles (EOM) in relation to innervation patterns. The orbital layer is composed of both singly-innervated (SIF) and multiply-innervated (MIF) fibres. The SIFs have the characteristics of twitch fibres, while the MIFs, in addition to possessing many small endings characteristic of tonic fibres, also have an en-plaque-like innervation in the endplate band resembling that of the adjacent SIFs. Myosin expression in MIFs and SIFs is unusual and varies systematically along the length of the fibres. Both SIFs and MIFs label with ALD180, but this labelling is absent in both fibre types in the endplate band region, where all fibres label with C14. Distally and also proximally to the endplate band, SIFs label with both ALD180 and C14, while the MIFs, innervated by many small, superficial endings in these regions, label with ALD180 only. This pattern of myosin expression could also be demonstrated in isolated fibres. The results are discussed in relation to the hypothesis that both populations of orbital layer fibres express constitutively both fast and the neonatal-like myosin, and that superimposed on this constitutive expression twitch or tonic innervation acts locally to selectively suppress either neonatal-like or fast myosin, respectively.
Similar content being viewed by others
References
Armstrong, R. B. &Phelps, R. O. (1984) Muscle fiber type composition of the rat hindlimb.Am. J. Anat. 171, 259–72.
Bach-Y-Rita, P. &Ito, F. (1966) In vivo studies on fast and slow muscle fibers in cat extraocular muscles.J. Gen. Physiol. 49, 1177–98.
Barany, M. (1967) ATPase activity of myosin correlated with speed of muscle shortening.J. Gen. Physiol. (Suppl.) 50, 197–218.
Barnard, R. J., Edgerton, V. R., Furukawa, T. &Peter, J. B. (1971) Histochemical, biochemical and contractile properties of red, white and intermediate fibers.Am. J. Physiol. 220, 410–414.
Barnard, E. A., Lyles, J. M. &Pizzey, J. A. (1982) Fibre types in chicken skeletal muscles and their changes in muscular dystrophy.J. Physiol. (Lond.) 331, 333–54.
Bondi, A. Y. &Chiarandini, D. J. (1979) Ionic basis for electrical properties of tonic fibres in rat extraocular muscles.J. Physiol. 295, 273–81.
Butler-Browne, G. S., Bugaisky, L. B., Cuenoud, S., Schwartz, K. &Whalen, R. G. (1982) Denervation of newborn rat muscles does not block the appearance of adult fast myosin heavy chain.Nature 299, 830–33.
Butler-Browne, G. S., Eriksson, P-O., Laurent, C. &Thornell, L-E. (1988) Adult human masseter muscle fibers express myosin isozymes characteristic of development.Muscle & Nerve 11, 610–20.
Butler-Brown, G. S. &Whalen, R. G. (1984) Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle.Dev. Biol. 102, 324–34.
Chiarandini, D. J. &Davidowitz, J. (1979) Structure and function of extraocular muscle fibers. InCurrent Topics in Eye Research, vol 1 (edited byZadunaisky, J. A. &Davson, H.), pp. 91–142. New York and London: Academic Press.
Chiarandini, D. J. &Stefani, E. (1979) Electrophysiological identification of two types of fibres in rat extraocular muscles.J. Physiol. 290, 453–65.
Close, R. I. &Luff, A. R. (1974) Dynamic properties of inferior rectus muscle of the rat.J. Physiol. 236, 259–70.
Davidowitz, J., Chiarandini, D. J., Philips, G. &Breinin, G. M. (1982) Morphological variation along multiply innervated fibers of rat extraocular muscles. InFunctional Basis of Ocular Motility Disorders (edited byLennerstrand, G., Zee, D. S. &Keller, E. L.), pp. 17–26. Oxford and New York: Pergamon Press.
Dhoot, G. K. (1986) Selective synthesis and degradation of slow skeletal myosin heavy chains in developing muscle fibers.Muscle & Nerve 9,155–64.
Guth, L. &Samaha, F. J. (1969) Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle.Exp. Neurol. 25, 138–52.
Guth, L. &Samaha, F. J. (1970) Procedure for the histochemical demonstration of actomyosin ATPase.Exp. Neurol. 28, 365–67.
Huxley, A. F. (1974) Muscular contraction.J. Physiol. 243, 1–43.
Jacoby, J., Chiarandini, D. J. &Stefani, E. (1989) Electrical properties of multiple innervated fibres in the orbital layer of rat extraocular msucles.J. Neurophysiol. 61, 116–25.
Jolesz, F. &Sreter, F. A. (1981) Development, innervation, and activity-pattern induced changes in skeletal muscle.Ann. Rev. Physiol. 43, 531–52.
Laing, N. G. &Lamb, A. H. (1983) The distribution of muscle fiber types in chick embryo wings transplanted to the pelvic region is normal.J. Embryol. Exp. Morphol. 78, 67–82.
Lyons, G. E., Haselgrove, J., Kelly, A. M. &Rubinstein, N. A. (1983) Myosin transitions in developing fast and slow muscles of the rat hindlimb.Differentiation 25, 168–75.
Mahdavi, V., Strehler, E. E., Periasamy, M., Wieczorek, D. F., Izumo, S. &Nadal-Ginard, B. (1986) Sarcomeric myosin heavy chain gene family: organization and pattern of expression.Med. Sci. Sports Exer. 18, 299–308.
Matsudaira, P. (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes.J. Biol. Chem. 262, 10035–38.
Moore, S. E., Hurko, O. &Walsh, F. S. (1984) Immunocytochemical analysis of fibre type differentiation in developing skeletal muscle.J Neuroimmunol. 7, 137–49.
Narusawa, M., Fitzsimons, R. B., Izumo, S., Nadal-Ginard, B., Rubinstein, N. A. &Kelly, A. M. (1987) Slow myosin in developing rat skeletal muscle.J. Cell Biol. 104, 447–59.
Pachter, B. R. (1983) Rat extraocular muscle. 1. Three dimensional cytoarchitecture, component fibre populations and innervation.J. Anat. 137, 143–59.
Pachter, B. R. (1984) Rat extraocular muscle. 3. Histochemical variability along the length of multiply-innervated fibers of the orbital surface layer.Histochem. 80, 535–38.
Pestronk, A. &Drachman, D. B. (1978) A new stain for quantitative measurement of sprounting at neuromuscular junctions.Muscle & Nerve 1, 70–4.
Pierobon Bormioli, S., Torresan, P., Sartore, S., Moschini, G. B. &Schiaffino, S. (1979) Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles.Invest. Ophthal. Vis. Sci. 18, 303–6.
Pierobon Bormioli, S., Sartore, S., Vitadello, M. &Schiaffino, S. (1980) ‘Slow’ myosins in vertebrate skeletal muscle. An immunofluorescence study.J. cell Biol. 85, 672–81.
Rajasekharan, K. N., Sivaramakrishnan, M. &Burke, M. (1987) Proximity and ligand-induced movement of interdomain residues in myosin subfragment 1 containing trapped MgADP and MgPPi probed by multifunctional cross-linking.J. Biol. Chem. 262, 11207–11210.
Reiser, P. J., Kasper, C. E., Greaser, M. L. &Moss, R. L. (1988) Functional significance of myosin transitions in single fibers of developing soleus muscle.Am. J. Physiol. 254 (Cell Physiol. 23): C605-C613.
Reiser, P. J., Moss, R. L., Giulian, G. G. &Greaser, M. L. (1985a) Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition.J. Biol. Chem. 260, 9077–80.
Reiser, P. J., Moss, R. L., Giulian, G. G. &Greaser, M. L. (1985b) Shortening velocity and myosin heavy chains of developing rabbit muscle fibers.J. Biol. Chem. 260, 14403–05.
Rubinstein, N. A. &Kelly, A. M. (1981) Development of muscle fiber specialization in the rat hindlimb.J. Cell Biol. 90, 128–144.
Rushbrook, J. I. &Stracher, A. (1979) Comparison of adult, embryonic, and dystrophic myosin heavy chains from chicken muscle by sodium dodecyl sulfate/ polyacrylamide gel electrophoresis and peptide mapping.Proc. Natl. Acad. Sci. USA 76, 4331–34.
Rushbrook, J. I., Weiss, C., Yao, T.-T. &Lin, J. (1988) Complexity of myosin species in the avian posterior latissimus dorsi muscle.J. Muscle Res. Cell Motil. 9, 552–62.
Salviati, G., Biasia, E. &Aloisi, M. (1986) Synthesis of fast myosin induced by fast ectopic innervation of rat soleus muscle is restricted to the ectopic endplate region.Nature 322, 637–39.
Sartore, S., Mascarello, F., Rowlerson, A., Gorza, L., Ausoni, S., Vianello, M. &Schiaffino, S. (1987) Fibre types in extraocular muscles: a new myosin isoform in the fast fibres.J. Musc. Res. Cell Motility.8, 161–72.
Shear, C. R., Bandman, E. &Rosser, B. W. C. (1988) Myosin heavy chain expression during development and following denervation of fast fibers in the red strip of the chicken pectoralis.Develop. Biol. 127, 326–37.
Stockdale, F. E. &Miller, J. B. (1987) The cellular basis of myosin heavy chain isoform expression during development of avian skeletal muscles.Develop. Biol. 123, 1–9.
Towbin, H., Staehelin, T. &Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications.Proc. Natl. Acad. Sci. USA 76, 4350–4.
Whalen, R. G., Sell, S. M., Butler-Browne, G. S., Schwartz, K., Bouveret, P. &Pinset-Harstrom, I. (1981) Three myosin heavy-chain isozymes appear sequentially in rat muscle development.Nature 292, 805–9.
Wieczorek, D. F., Periasamy, M., Butler-Browne, G. S., Whalen, R. G. &Nadal-Ginard, B. (1985) Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature.J. Cell Biol. 101, 618–29.
Yellin, H. (1969) Unique intrafusal and extraocular muscle fibers exhibiting dual actomyosin ATPase activity.Exp. Neurol. 25, 153–63.
Zhang, Y. &Shafiq, S. A. (1987) Differentiation of the anterior labissimus dorsi muscle of the chicken examined by anti-myosin monoclonal antibodies.J. Exp. Zool. 243, 51–62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jacoby, J., Ko, K., Weiss, C. et al. Systematic variation in myosin expression along extraocular muscle fibres of the adult rat. J Muscle Res Cell Motil 11, 25–40 (1990). https://doi.org/10.1007/BF01833323
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01833323