Summary
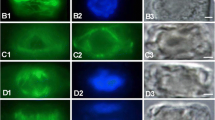
Nongrowing, two-celled protonemata of the fernAdiantum capillus-veneris L. resume tip growth within the apical cell upon irradiation with red light. In this study, the phenomenon of growth resumption was analyzed with reference to changes in cytoskeletal organization. Continuous observations of apical cells with time lapse video-microscopy revealed that the nucleus migrated toward the tip ca. 1.9 h after the onset of red light, much earlier than the initiation of tip growth, which took place ca. 8.5 h after irradiation. Cytoskeletal organization was observed at various time points during growth resumption by fluorescent staining of microfilaments (MFs) and microtubules (MTs) with rhodamine-phalloidin and anti-tubulin antibodies. At 2 h after red-light irradiation, endoplasmic MF and MT strands appeared at the apical end of nucleus. These strands extended into the apical endoplasm, where filaments were rare prior to irradiation. Many fine filaments branched from the strands to the cell periphery, including the cortex of the apical-dome region. At this time, cortical circular arrays of MTs and MFs, normally found in the growing apex of protonemal cells, were absent. Both MT and MF circular arrays appeared during the resumption of tip growth concomitantly. The half-maximum appearance of MT and MF circular arrays within a population occurred at 5.4 h and 5.8 h after red-light irradiation, respectively. Thus, the process of red-light-induced resumption of tip growth in fern protonemal cell is composed of a series of events. These events include: (1) the appearance of strands extending from the nucleus toward the apical cortex and the migration of nucleus toward the apex; (2) the formation of circular MT and MF arrays at the sub-apical cortex; and (3) the initiation of cell growth at the apex. These results reflect the significant roles of MF and MT cytoskeleton in the resumption of tip growth.
Similar content being viewed by others
Abbreviations
- MBS:
-
m-maleimidobenzoic acid N-hydroxysuccinimide ester
- MF:
-
microfilament
- MT:
-
microtubule
References
Doonan JH (1991) The cytoskeleton and moss morphogenesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 289–301
Henry C, Jordan JR, Kropf DL (1996) Localized membrane-wall adhesions inPelvetia zygotes. Protoplasma 190: 39–52
Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48: 461–491
Kadota A, Furuya M (1977) Apical growth of protonemata inAdiantum capillus-veneris I: red far-red reversible effect on growth cessation in the dark. Dev Growth Differ 19: 357–365
— — (1981) Apical growth of protonemata inAdiantum capillusveneris IV: phytochrome-mediated induction in non-growing cells. Plant Cell Physiol 22: 629–638
—, Wada M (1989) Circular arrangement of cortical F-actin around the subapical region of a tip-growing fern protonemal cell. Plant Cell Physiol 30: 1183–1186
— — (1992a) Reorganization of the cortical cytoskeleton in tip-growing fern protonemal cells during phytochrome-mediated phototropism and blue light-induced apical swelling. Protoplasma 166: 35–41
— — (1992b) The circular arrangement of cortical microtubules around the subapex of tip-growing fern protonemata is sensitive to cytochalasin B. Plant Cell Physiol 33: 99–102
— — (1995) Cytoskeletal aspects of nuclear migration during tip-growth in the fernAdiantum protonemal cell. Protoplasma 188: 170–179
Kagawa T, Kadota A, Wada M (1992) The junction between the plasma membrane and the cell wall in fern protonemal cells, as visualized after plasmolysis, and its dependence on arrays of cortical microtubules. Protoplasma 170: 186–190
Kropf DL (1992) Role of the cytoskeleton in cellular morphogenesis of zygotes of fucoid algae. In: Menzel D (ed) The cytoskeleton of the algae. CRC Press, Boca Raton, pp 79–92
—, Bisgrove SR, Hable WE (1998) Cytoskeletal control of polar growth in plant cells. Curr Opin Cell Biol 10: 117–122
Li Y-Q, Moscatelli A, Cai G, Cresti M (1997) Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. Int Rev Cytol 176: 133–199
Murata T, Wada M (1989) Re-organization of microtubules during preprophase band development inAdiantum protonemata. Protoplasma 151: 73–80
—, Kadota A, Hogetsu T, Wada M (1987) Circular arrangement of cortical microtubules around the subapical part of a tip-growing fern protonema. Protoplasma 141: 135–138
Nagai R (1993) Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol 145: 251–310
Quader H, Schnepf E (1989) Actin filament array during side branch initiation in protonema cells of the mossFunaria hygrometrica: an actin organizing center at the plasma membrane. Protoplasma 151: 167–170
Schnepf E (1986) Cellular polarity. Annu Rev Plant Physiol 37: 23–47
Wada M, Furuya M (1972) Phytochrome action on the timing of cell division inAdiantum gametophytes. Plant Physiol 49: 110–113
Wada M, Murata T (1991) The cytoskeleton in fern protonemal growth in relation to photomorphogenesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 277–288
—, Mineyuki Y, Kadota A, Furuya M (1980) The changes of nuclear position and distribution of circumferentially aligned cortical microtubules during the progression of cell cycle inAdiantum protonemata. Bot Mag Tokyo 93: 237–245
—, Murata T, Shibata M (1990) Changes in microtubule and microfibril arrangement during polarotropism inAdiantum protonemata. Bot Mag Tokyo 103: 391–401
—, Nozue K, Kadota A (1998) Cytoskeletal pattern changes during branch formation in a centrifugedAdiantum protonema. J Plant Res 111: 53–58
Williamson RE (1993) Organelle movements. Annu Rev Plant Physiol Plant Mol Biol 44: 181–202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadota, A., Yoshizaki, N. & Wada, M. Cytoskeletal changes during resumption of tip growth in nongrowing protonemal cells of the fernAdiantum capillus-veneris L.. Protoplasma 207, 195–202 (1999). https://doi.org/10.1007/BF01283000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01283000