Summary
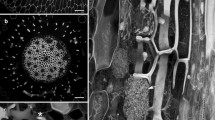
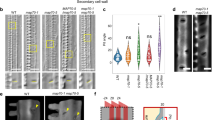
A correlative study, using indirect immunofluorescence microscopy (IIF) of anti-α-tubulin stained sections and transmission electron microscopy (TEM), gave details of the involvement of cortical microtubules (CMTs) in the development of bordered pits in secondary xylem vessel elements ofAesculus hippocastanum L. In addition, aspects of wall cytochemistry were studied during this process using the Thiéry (PATAg) test, immunolocalization with the monoclonal antibodies JIM5 and JIM7, and a range of other cytochemical procedures. IIF showed that the alternately-arranged pits are pre-figured as perforations within a reticulum of randomly-oriented CMTs before any secondary wall thickening is obvious. Each incipient pit border is subsequently delimited by a circle of CMTs whose diameter decreases as deposition of secondary wall takes place around the perforation. These IIF observations are corroborated by a parallel TEM study. During the period of bordered pit formation, the secondary walls of the cell are lignifying. At maturity, however, the pit membrane is unlignified and continues to stain strongly with the monoclonal antibody JIM5, a marker of primary, “juvenile” wall. The results are discussed in terms of the relationship of the CMT cytoskeleton with development of bordered pits.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- CMT(s):
-
cortical micro-tubule(s)
- EGTA:
-
ethylene glycol-bis-(ß-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- FITC:
-
fluorescein isothiocyanate
- IIP:
-
indirect immunofluorescence
- MAP:
-
microtubule-associated protein
- mf(s):
-
wall microfibril(s)
- MTSB:
-
microtubule-stabilising buffer
- PATAg:
-
periodic acid-thiocarbohydrazide-silver proteinate
- PBS:
-
Phosphate-buffered saline
- PIPES:
-
piperazine-N,N′-bis-[2-ethylsulphonic acid]
- SVS:
-
secondary vascular system
- TEM:
-
transmission electron microscopy
References
Abe H, Funada R, Imaizumi H, Ohtani J, Fukazawa K (1995a) Dynamic changes in the arrangement of cortical microtubules in conifer tracheids during differentiation. Planta 197: 418–421
— —, Ohtani J, Fukazawa K (1995b) Changes in the arrangement of microtubules and microfibrils in differentiating conifer tracheids during the expansion of cells. Ann Bot 75: 305–310
Apostolakos P, Galatis B, Panteris E (1991) Microtubules in cell morphogenesis and intercellular space formation inZea mays lead mesophyll andPilea cadieri epithem. J Plant Physiol 137: 591–601
Baluška F, Barlow PW, Hauskrecht M, Kubica Š, Parker JS, Volkmann D (1995) Microtubule arrays in maize root cells. Interplay between the cytoskeleton, nuclear organization and post-mitotic cellular growth patterns. New Phytol 130: 177–192
Barnett JR (1981) Secondary xylem cell development. In: Barnett JR (ed) Xylem cell development. Castle House Publications, Tunbridge Wells, pp 47–95
— (1992) Reactivation of the cambium inAesculus hippocastanum L.: a transmission electron microscope study. Ann Bot 70: 169–177
— (1995) Ultrastructural factors affecting xylem differentiation. In: Iqbal M (ed) Vascular cambial derivatives. Gebrüder Borntraeger, Berlin, pp 107–130
—, Harris JM (1975) Early stages of bordered pit formation in radiata pine. Wood Sci Technol 9: 233–241
Burgess J, Linstead P (1984) Comparison of tracheary element differentiation in intact leaves and isolated mesophyll cells ofZinnia elegans. Micron 15: 153–160
Catesson A-M (1974) Cambial cells. In: Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill Book, London, pp 358–390
Chaffey NJ (1985) Structure and function in the grass ligule: the membranous ligule ofLolium temulentum L. as a secretory organ. Protoplasma 127: 128–132
— (1994) Structure and function of the membranous grass ligule: a comparative study. Bot J Linn Soc 116: 53–69
— (1996) Structure and function of the root cap ofLolium temulentum L. (Poaceae): parallels with the ligule. Ann Bot 78: 3–13
—, Pearson JA (1985) Presence of tyloses at the blade/sheath junction in senescing leaves ofLolium temulentum L. Ann Bot 56: 761–770
—, Barlow PW, Barnett JR (1994) The microtubular cytoskeleton of the vascular cambium and its derivatives in the root ofAesculus hippocastanum L. (Hippocastanaceae). IAWA J 15: 201
— — — (1996a) Microtubular cytoskeleton of vascular cambium and its derivatives in roots ofAesculus hippocastanum L. (Hippocastanaceae). In: Donaldson LA, Butterfield BG, Singh PA, White-house LJ (eds) Recent advances in wood anatomy. New Zealand Forest Research Institute, Rotorua, pp 171–183
- - - (1996b) Seeing the wood in the trees. BBSRC Business, October: 16–18
- - - (1997a) Cortical microtubules rearrange during differentiation of vascular cambial derivatives, microfilaments do not. Trees (in press)
- Barnett JR, Barlow PW (1997b) Endomembranes, cytoskeleton and cell walls: aspects of the ultrastructure of the vascular cambium of taproots ofAesculus hippocastanum L. (Hippocastanaceae). Int J Plant Sci (in press)
Clark G (ed) (1981) Staining procedures, 4th edn. Williams and Wilkins, Baltimore
Côté WA Jr (1958) Electron microscope studies of pit membrane structure: implications in seasoning and preservation of wood. For Prod 18: 296–301
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol 10: 153–180
Exley RR, Butterfield BG, Meylan BA (1974) Preparation of wood specimens for the scanning electron microscope. J Microsc 101: 21–30
Falconer MM, Seagull RW (1988) Xylogenesis in tissue culture III: continuing wall deposition during tracheary element development. Protoplasma 144: 10–16
Fukuda H (1994) Redifferentiation of single mesophyll cells into tracheary elements. Int J Plant Sci 155: 262–271
Giddings TH Jr, Staehelin LA (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 85–99
Goosen de-Roo L, van Spronsen PC (1978) Electron microscopy of the active cambial zone ofFraxinus excelsior L. IAWA Bull 1978/4: 59–64
Gunning BBS, Hardham AR (1979) Microtubules and morphogenesis in plants. Endeavour NS 3: 112–117
Hepler PK (1981) Morphogenesis of tracheary elements and guard cells. In: Kiermayer O (ed) Cytomorphogenesis in plants. Springer, Wien New York, pp 327–347
Imamura Y, Harada H (1973) Electron microscopic study on the development of the bordered pit in coniferous tracheids. Wood Sci Technol 7: 189–205
Ishida K, Katsumi C (1992) Effects of gibberellin and abscisic acid on the microtubule orientation in hypocotyl cells of light-grown cucumber seedlings. Int J Plant Sci 153: 155–163
Jung G, Wernicke W (1990) Cell-shaping and microtubules in developing mesophyll of wheat (Triticum aestivum L.). Protoplasma 153: 141–148
Knox JP (1992) Molecular probes for the plant cell surface. Protoplasma 167: 1–9
— (1993) The role of cell surface glycoproteins in differentiation and morphogenesis. In: Battey NH, Dickinson HG, Hetherington AM (eds) Posttranslational modifications in plants. Cambridge University Press, Cambridge, pp 267–283
—, Linstead PJ, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521
Ledbetter MC, Porter KR (1963) A “microtubule” in plant cell fine structure. J Cell Biol 19: 239–250
— — (1964) Morphology of microtubules of plant cells. Science 144: 872–874
Lloyd CW (ed) (1982) The cytoskeleton in plant growth and development. Academic Press, London
— (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu Rev Plant Physiol 38: 119–139
— (1991) The cytoskeletal basis of plant growth and form. Academic Press, London
—, Shaw PJ, Warn RM, Yuan M (1996) Gibberellic-acid-induced reorientation of cortical microtubules in living plant cells. J Microsc 181: 140–144
—, Slabas AR, Powell AJ, MacDonald G, Badley RA (1979) Cytoplasmic microtubules of higher plant cells visualised with antitubulin antibodies. Nature 279: 239–241
Machado RD, Schmid R (1964) Observations in the structure of pit membranes in hardwood cells. Proc Third Eur Reg Conf Electron Microsc: 163–164
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termacarphi Pty, Melbourne
Panshin AJ, de Zeeuw C (1980) Textbook of wood technology, 4th edn. McGraw-Hill, New York
Panteris E, Apostolakos P, Galatis B (1993) Microtubule organization, mesophyll cell morphogenesis, and intercellular space formation inAdiantum capillus veneris leaflets. Protoplasma 172: 97–110
— — — (1994) Sinuous ordinary epidermal cells: behind several patterns of waviness, a common morphogenetic mechanism. New Phytol 127: 771–780
Porter KR (1966) Cytoplasmic microtubules and their functions. In: Wolstenholme GEW, O'Connor M (eds) CIBA Foundation Symposium: principles of biomolecular organization. J & A Churchill, London, pp 308–356
Prodhan AKMA, Funada R, Ohtani J, Abe H, Fukazawa K (1995) Orientation of microfibrils and microtubules in developing tension-wood fibres of Japanese ash (Fraxinus mandshurica var.japonica). Planta 196: 577–585
Robards AW, Humpherson PG (1967) Microtubules and angiosperm bordered pit formation. Planta 77: 233–238
Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced microtubule reorientations mediated by helical arrays. Planta 164: 439–447
Roberts K (1990) Structures at the plant cell surface. Curr Opin Cell Biol 2: 920–928
Robinson DG, Quader H (1982) The microtubule-microfibril syndrome. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 109–126
Sakaguchi S, Hogetsu T, Kara N (1988) Arrangement of cortical microtubules in the shoot apex ofVinca major L. Planta 175: 403–411
Schmid A-MR, Eberwein RK, Hesse M (1996) Pattern morphogenesis in cell walls of diatoms and pollen grains: a comparison. Protoplasma 193: 144–173
Schmid R (1965) The fine structure of pits in hardwoods. In: Côté WA Jr (ed) Cellular ultrastructure of woody plants. Syracuse University Press, Syracuse, NY, pp 291–304
Seagull RW (1989) The plant cytoskeleton. Crit Rev Plant Sci 8: 131–167
— (1993) Cytoskeletal involvement in cotton fiber growth and development. Micron 24: 643–660
Siau JF (1995) Wood: influence of moisture on physical properties. Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University, Blacksburg, VA
Staiger CJ (1994) Indirect immunofluorescence: localization of the cytoskeleton. In: Freeling M, Walbot V (eds) The maize handbook. Springer, Berlin Heidelberg New York Tokyo, pp 140–149
Staxén I, Klimaszewska K, Bornmann CH (1994) Microtubular organization in protoplasts and cells of somatic embryo-regenerating and non-regenerating cultures ofLarix. Physiol Plant 91: 680–686
Thiéry J-P (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6: 987–1018
Torrey JG (1975) Tracheary element formation from single isolated cells in culture. Physiol Plant 35: 158–165
—, Fosket DE, Kepler PK (1971) Xylem formation: a paradigm of cytodifferentiation in higher plants. Am Scient 59: 338–352
Uehara K, Hogetsu T (1993) Arrangement of cortical microtubules during formation of bordered pit in the tracheids ofTaxus. Protoplasma 172: 145–153
Wardrop AB (1954) The mechanism of surface growth involved in the differentiation of fibres and tracheids. Aust J Bot 2: 165–175
Wick SM, Cho S-O, Mundelius AR (1989) Microtubule deployment within plant tissues: fluorescence studies of intact mesophyll and epidermal cells. Cell Biol Int Rep 13: 95–106
Wyatt SE, Carpita NC (1993) The plant cytoskeleton-cell-wall continuum. Trends Cell Biol 3: 413–417
Yuan M, Warn RM, Shaw PJ, Lloyd CW (1995) Dynamic microtubules under the radial and outer tangential walls of microinjected pea epidermal cells observed by computer reconstruction. Plant J 7: 17–23
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaffey, N.J., Barnett, J.R. & Barlow, P.W. Cortical microtubule involvement in bordered pit formation in secondary xylem vessel elements ofAesculus hippocastanum L. (Hippocastanaceae): A correlative study using electron microscopy and indirect immunofluorescence microscopy. Protoplasma 197, 64–75 (1997). https://doi.org/10.1007/BF01279885
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279885