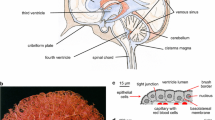
Summary
The potential for transcytosis (endocytosis → intracellular transport → exocytosis) of protein and membrane events associated with fluid phase and adsorptive endocytic processes within epithelia of the choroid plexus [blood-cerebrospinal fluid (CSF) barrier] were investigated in mice injected intravenously or into the lateral cerebral ventricle with native horseradish peroxidase (HRP) or the lectin wheatgerm agglutinin (WGA) conjugated to HRP. WGA binds to specific cell surface oligosaccharides and enters cells by the process of adsorptive endocytosis; native HRP is taken into cells non-specifically by fluid phase endocytosis. The lysosomal system of organelles and the endoplasmic reticulum, identified by enzyme cytochemical markers applied to choroid epithelia, were analysed for possible participation in transcytosis and compared to epithelial organelles harbouring the exogenous tracer proteins. Blood-borne native HRP was endocytosed readily by choroid epithelia whereas WGA-HRP was not, perhaps because WGA-HRP does not escape fenestrated endothelia as easily as native HRP. The blood-borne proteins incorporated within endocytic vesicles by choroid epithelia were directed to endosomes (prelysosomes) and secondary lysosomes (e.g. tubules, multivesicular/dense bodies) for eventual degradation and did not reach the apical/microvillus surface. Both CSF-borne native HRP and WGA-HRP entered choroid epithelia within endocytic vesicles derived from the microvillus border. Native HRP, ultimately sequestered within endosomes and secondary lysosomes, failed to undergo transcytosis through the epithelia into the basolateral clefts. Conversely, CSF-borne WGA-HRP was transported through the epithelia and released into the basolateral clefts within 10 min post-injection. The lectin conjugate labelled epithelial vesicles, endosomes, secondary lysosomes and, at 30 min post-injection, the transmost saccule of the Golgi complex which exhibits acid hydrolase activity. Tubular profiles, related either to the endosome apparatus or to the lysosomal system, and the endoplasmic reticulum did not appear involved in the transcytotic pathway. The data suggest that CSF-borne protein entering the choroid epithelium by adsorptive endocytosis can undergo rapid transcytosis through the cell. The results provide insight to transcytotic pathways utilizing vesicles, the endosomal apparatus, and the Golgi complex within the choroid epithelium for circumventing the blood-CSF barrier. Hypothesized membrane events and morphological associations among constituents of the endomembrane system within the choroid epithelium are summarized diagrammatically.
Similar content being viewed by others
References
Balin, B. J. &Broadwell, R. D. (1987) Lectin-labeled membrane is transferred to the Golgi complex in mouse pituitary cellsin vivo, Journal of Histochemistry and Cytochemistry 35 489–98.
Balin, B. J., Broadwell, R. D. &Salcman, M. (1987) Tubular profiles do not form transendothelial channels through the blood-brain barrier.Journal of Neurocytology 16, 721–35.
Balin, B. J., Broadwell, R. D., Salcman, M. &Elkalliny, M. (1986) Avenues for entry of peripherally administered protein to the CNS in mouse, rat and monkey.Journal of Comparative Neurology 251, 260–80.
Becker, N. H. &Almazon, R. (1968) Evidence for the functional polarization of micropinocytotic vesicles in the rat choroid plexus,Journal of Histochemistry and Cytochemistry 16, 278–80.
Becker, N. H., Novikoff, A. B. &Zimmerman, H. M. (1967) Fine structure observations of the uptake of intravenously injected peroxidase by the rat choroid plexus.Journal of Histochemistry and Cytochemistry 15, 160–5.
Bennett, G., Kan, F. W. K. &O'shaughnessy, D. (1981) The site of incorporation of sialic acid residues into giycoproteins and the subsequent fate of these-molecules in various rat and mouse cell types as shown by radioautography after injection of (3H)N-acetylmannosamine. II. Observations in tissues other than liver.Journal of Cell Biology 88, 16–31.
Bennett, G. &O'shaughnessy, D. (1981) The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of (3H)N-acetylmannosamine. I. Observations in hepatocytes.Journal of Cell Biology 88, 1–15.
Bergeron, J. J., Cruz, J., Khan, M. N. &Posner, B. I. (1985) Uptake of insulin and other ligands into receptorrich endocytic components of target cells: the endosomal apparatus.Annual Review of Physiology 47, 383–404.
Bouchaud, C. &Bouvier, D. (1978) Fine structure of tight junctions between rat choroidal cells after osmotic opening induced by urea and sucrose.Tissue and Cell 10, 331–42.
Brightman, M. W. (1968) The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice.Progress in Brain Research 29, 19–37.
Brightman, M. W. (1975) Ultrastructural characteristics of adult choroid plexus: Relation to the blood-cerebrospinal fluid barrier to proteins. InThe Choroid Plexus in Health and Disease (edited byNetsky, M. G. &Shuangshati, S.), pp. 86–112. Charlottesville: University Virginia Press.
Brightman, M. W., Klatzo, I., Olsson, Y. &Reese, T. S. (1970) The blood-brain barrier to proteins under normal and pathological conditions.Journal of the Neurological Sciences 10 215–39.
Brightman, M. W. &Reese, T. S. (1969) Junctions between intimately opposed cell membrane in the vertebrate brain.Journal of Cell Biology 40, 648–77.
Broadwell, R. D. (1988) Movement of macromolecules across the blood-brain barrier. InThe 16th Princeton Conference on Cerebrovascular Diseases (edited byGinsburg, M.). New York: Raven Press (in press).
Broadwell, R. D. &Balin, B. J. (1985) Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheatgerm agglutininhorseradish peroxidasein vivo.Journal of Comparative Neurology 242, 632–50.
Broadwell, R. D., Balin, B. J. &Cataldo, A. M. (1987a) Fine structure and cytochemistry of the mammalian median eminence. InCircumventricular Organs and Body Fluids (edited byGross, P. M.), pp. 61–85. Boca Raton, Florida: CRC Press.
Broadwell, R. D., Balin, B. J. &Salcman, M. (1987b) Polarity of the blood-brain barrier to the endocytosis of protein.Wiss. Z. Karl-Marx-Univ. Leipzig, Math.-Naturwiss. R. 36, 170–4.
Broadwell, R. D., Balin, B. J. &Salcman, M. (1988) Transcytotic pathway for blood-borne protein through the blood-brain barrier.Proceedings of the National Academy of Sciences USA 85, 632–6.
Broadwell, R. D., Balin, B. J., Salcman, M. &Kaplan, R. S. (1983) Brain-blood barrier? Yes and no.Proceedings of the National Academy of Sciences USA 80, 7352–6.
Broadwell, R. D. &Brightman, M. W. (1979) Cytochemistry of undamaged neurons transporting exogenous proteinin vivo.Journal of Comparative Neurology 185, 31–74.
Broadwell, R. D. &Brightman, M. W. (1983) Horseradish peroxidase: A tool to study the neuroendocrine cell and other peptide-secreting cells.Methods in Enzymology 103, 187–218.
Broadwell, R. D., Brightman, M. W. &Oliver, C. (1980) Neuronal transport of acid hydrolases and peroxidase within the lysosomal system of organelles: Involvement of agranular reticulum-like cisterns.Journal of Comparative Neurology 190, 519–32.
Broadwell, R. D. &Cataldo, A. M. (1984) The neuronal endoplasmic reticulum. Its cytochemistry and contribution to the endomembrane system. II. Axons and terminals.Journal of Comparative Neurology 230, 231–48.
Broadwell, R. D., Charlton, H. M., Balin, B. J. &Salcman, M. (1987c) Angioarchitecture of the CNS, pituitary gland, and intracerebral grafts revealed with peroxidase cytochemistry.Journal of Comparative Neurology 260, 47–62.
Broadwell, R. D. &Oliver, C. (1983) An enzyme cytochemical study of the endocytic pathways in anterior pituitary cells of the mousein vivo.Journal of Histochemistry and Cytochemistry 31, 325–35.
Broadwell, R. D. &Salcman, M. (1981) Expanding the definition of the blood-brain barrier to protein.Proceedings of the National Academy of Sciences (USA) 78, 7820–4.
Brown, M. S., Anderson, R. G. W., Barr, S. K. &Goldstein, J. L. (1982) Recycling of cell surface receptors: Observations from the LDL receptor system.Cold Spring Harbor Symposium on Quantitative Biology 46, 713–20.
Burwen, J., Barker, M. E., Goldman, I. S., Hradek, G. T., Roper, S. E. &Jones, A. L. (1984) Transport of epidermal growth factor by rat liver: Evidence for a non-lysosomal pathway.Journal of Cell Biology 99, 1259–65.
Cataldo, A. M. &Broadwell, R. D. (1986a) Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. I. Neurons and glia.Journal of Electron Microscopy Technique 3, 413–37.
Cataldo, A. M. &Broadwell, R. D. (1986b) Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes.Journal of Neurocytology 15, 511–24.
Coomber, B. L. &Stewart, P. A. (1986) Three dimensional reconstruction of vesicles in endothelium of blood-brain barrier versus highly permeable microvessels.Anatomical Record 215, 256–61.
Dautry-Varsat, A. &Lodish, H. F. (1983) The Golgi complex and the sorting of membrane and secreted proteins.Trends in Neuroscience 6, 484–90.
Dautry-Varsat, A. &Lodish, H. F. (1984) How receptors bring proteins and particles into cells.Scientific American 250, 52–8.
Davis, D. A. &Milhorat, T. H. (1975) The blood-brain barrier of the rat choroid plexus.Anatomical Record 181, 779–87.
De Bruyn, P. P. H., Michelson, S. &Becker, R. P. (1975) Endocytosis, transfer tubules, and lysosomal activity in myeloid sinusoidal endothelium.Journal of Ultrastructure Research 53, 133–51.
Doty, S. B., Smith, C. E., Hand, A. R. &Oliver, C. (1977) Inorganic trimetaphosphatase as a histochemical marker for lysosomes in light and electron microscopy.Journal of Histochemistry and Cytochemistry 25, 1381–4.
Dunn, W. A., Connolly, T. P. &Hubbard, A. L. (1986) Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway.Journal of Cell Biology 102, 24–36.
Dunn, W. A. &Hubbard, A. L. (1984) Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: Ligand and receptor dynamics.Journal of Cell Biology 98, 2148–59.
Gomori, G. (1952)Microscopic Histochemistry: Principles and Practice, pp. 189–273. Chicago: University of Chicago Press.
Gonatas, J. K. &Avrameas, S. (1973) Detection of plasma membrane carbohydrates with lectin peroxidase conjugates.Journal of Cell Biology 59, 436–45.
Gonatas, J. K., Steiber, A., Hickey, W. F., Herbert, S. H. &Gonatas, J. O. (1984) Endosomes and Golgi vesicles in adsorptive and fluid phase endocytosis.Journal of Cell Biology 99, 1379–90.
Graham, R. C. &Karnovsky, M. J. (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: Ultrastructural cytochemistry by a new technique.Journal of Histochemistry and Cytochemistry 14, 291–302.
Helenius, A., Mellman, I., Wall, D. &Hubbard, A. (1983) Endosomes.Trends in Biochemical Science 8, 245–9.
Mesulam, M.-M. (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents.Journal of Histochemistry and Cytochemistry 26, 106–17.
Møllgård, K. &Saunders, N. R. (1975) Complex tight junctions of epithelial and endothelial cells in early foetal brain.Journal of Neurocytology 4, 453–68.
Møllgård, K. &Saunders, N. R. (1977) A possible transepithelial pathway via endoplasmic reticulum in foetal sheep choroid plexus.Proceedings of the Royal Society of London Series B 199, 321–6.
Mueller, S. C. &Hubbard, A. L. (1986) Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: Receptorpositive and receptor-negative endosomes.Journal of Cell Biology 102, 932–42.
Novikoff, A. B. (1963) Lysosomes in the physiology and pathology of cells: Contributions of staining methods. InCiba Foundation Symposium on Lysosomes (edited byDeReuck, A. V. S. &Cameron, M. P.), pp. 35–77. Boston: Little, Brown & Co.
Oliver, C. (1980) Cytochemical localization of acid phosphatase and trimetaphosphatase activities in exocrine acinar cells,Journal of Histochemistry and Cytochemistry 28, 78–81.
Oliver, C. (1983) Characterization of basal lysosomes in exocrine acinar cells.Journal of Histochemistry and Cytochemistry 31, 1209–16.
Rodewald, R. (1973) Intestinal transport of antibodies in the newborn rat.Journal of Cell Biology 58, 189–211.
Rodewald, R. &Abrahamson, D. R. (1982) Receptormediated transport of IgG through the intestinal epithelium of the neonatal rat.Ciba Foundation Symposium 92, 209–32.
Rodewald, R. &Kraehenbuhl, J.-P. (1984) Receptormediated transport of IgG.Journal of Cell Biology 99, 159s-164s.
Steinman, R. M., Mellman, I. S., Muller, W. A. &Cohn, Z. A. (1983) Endocytosis and the recycling of plasma membrane.Journal of Cell Biology 96, 1–27.
Tangoren, M., Broadwell, R. D., Moriyama, E., Oliver, C. &Wolf, A. (1988) How significant is the blood-brain barrier?Abstracts of the Society for Neuroscience 14, 617.
Van Deurs, B. (1976) Choroid plexus absorption of horseradish peroxidase from the cerebral ventricles.Journal of Ultrastructure Research 55, 400–16.
Van Deurs, B. (1978) Horseradish peroxidase uptake into the rat choroid plexus epithelium, with special reference to the lysosomal system.Journal of Ultrastructure Research 62, 155–67.
Van Deurs, B., Moller, M. &Amtorp, O. (1978) Uptake of horseradish peroxidase from CSF into the choroid plexus of the rat with special reference to transepithelial transport.Cell and Tissue Research 187, 15–23.
Van Deurs, B., Von Bulow, F. &Moller, M. (1981) Vesicular transport of cationized ferritin by the epithelium of rat choroid plexus.Journal of Cell Biology 89, 131–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Balin, B.J., Broadwell, R.D. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. I. Choroid plexus and the blood-cerebrospinal fluid barrier. J Neurocytol 17, 809–826 (1988). https://doi.org/10.1007/BF01216708
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01216708