Abstract
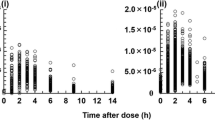
The pharmacokinetics of Cyclosporin A (CyA, SandimmuneR) was studied in 12 healthy male volunteers after oral dosing of 350 mg, 700 mg, and 1400 mg as a drinking solution. Blood samples were collected over 96 hr and analyzed by high pressure liquid chromatography. Concentration data were evaluated with model-independent and model-based linear pharmacokinetic concepts. Individual CyA concentration-time profiles in whole blood were well described by a two-compartment open model with zero-order absorption for all three doses. Comparison of pharmacokinetic parameters across doses indicates that both absorption and disposition are dose-dependent. Nonlinear disposition is suggested by the significant increase of the terminal half-life from 8.9±4.9hr to 11.9±4.9hr (mean±SD) after a 350 mg and a 1400 mg dose, respectively. Changes in the metabolic activity of the liver with concentration might be responsible for this phenomenon. In addition, the modeling approach indicated that bioavailability decreases with increasing dose. Moreover, the dependence of the rate of CyA absorption (zero-order rate constant) versus dose was well described by a hyperbola. The limited solubility of the drug in the gastrointestinal tract might be responsible for this behavior. The lag time (0.2–0.8 hr) was independent of dose. This value is similar to the time of gastric emptying in fasting volunteers. The duration of absorption for 11 of 12 subjects was in the range 2.5–3.5 hr over all doses and agrees well with the small intestine transit time. Some subjects showed a marked secondary peak at one or two doses, which could be adequately fitted by a model with two successive zero-order inputs. This double-peak behavior was ascribed to the influence of the food on gastric emptying. Dose dependency of disposition and absorption counterbalance each other in the usual dose range. This leads to an almost proportional increase of area under the blood CyA concentration-time profile with increasing dose.
Similar content being viewed by others
References
R. J. Ptachcinski, R. Venkataramanan, and G. J. Burckart. The clinical pharmacokinetics of cyclosporine.Clin. Pharmacokin. 11:107–132 (1986).
R. J. Ptachcinski, R. Venkataramanan, G. J. Burckart, J. T. Rosenthal, R. J. Taylor, and T. R. Hakala. Dose-dependent absorption of cyclosporineDrug Intell. Clin. Pharm. 19:450 (1985).
B. D. Kahan. Individualization of cyclosporine therapy using pharmacokinetic and pharmacodynamic parameters. Transplantation40:457–476 (1985).
J. Grevel, E. Nüesch, E. Abisch, and K. Kutz. Pharmacokinetics of oral cyclosporin A in healthy subjects.Eur. J. Clin. Pharmacol. 31:211–216 (1986).
C. R. Abolin, H. F. Schran, D. W. Bitz, and S. Solar-Yohay.A Study of the Dose-Bioavailbility Relationship of Sandimmune Oral Solution in Normal Volunteers (Study No. 50), Sandoz Inc., East-Hanover, 1982.
D. Du Bois and E. F. Du Bois. A formula to estimate the approximate surface area if height and weight be known.Arch. Intern. Med. 17:863 (1916).
H. T. Smith and W. T. Robinson. Semi-automated liquid chromatographic method for the determination of cyclosporine in plasma and blood using column switching.J. Chromatog. 305:353–362 (1984).
R. J. Ptachcinski, R. Venkataramanan, G. J. Burckart, J. Gray, D. H. Van Thiel, A. Sanghvi, and J. T. Rosenthal. Cyclosporine kinetics in normal volunteers.J. Clin. Pharmacol. 27:243–248 (1987).
J. P. Reymond. In vitro in vivo Modelle zur Absorption von Cyclosporin A. Ph.D. dissertation, University of Basel, 1986.
L. B. Sheiner. ELSFIT, a program for the extended least squares fit to individual pharmacokinetic data. A technical report of the Division of Clinical Pharmacology, University of California, 1980.
D. Perrier and M. Mayersohn. Noncompartmental determination of the steady-state volume of distribution for any mode of administration.J. Pharm. Sci. 71:372–373 (1982).
P. R. Gwilt, M. C. Pankaskie, J. E. Thornburg, R. Zustiak, and D. R. Shoenthal. Pharmacokinetics of methylprylon following a single oral dose.J. Pharm. Sci. 74:1001–1003 (1985).
RS/1Version 12.00. BBN Research Systems, Cambridge, MA, 1984.
E. M. Landaw and J. J. Distefano III. Multiexponential, multicompartmental, and noncompartmental modeling II. Data analysis and statistical considerations.Am. J. Physiol. 246:R665-R677 (1984).
L. J. Lesko, J. Minor, D. Yocum, T. Emm, and J. H. Klippel. Pharmacokinetics of cyclosporine in patients with rheumatoid arthiritis.Clin. Pharmacol. Ther. 39:207 (1986).
S. Øie, T. W. Guentert, and T. N. Tozer. Effect of saturable binding on the pharmacokinetics of drugs: a simulation.J. Pharm. Pharmacol. 32:471–477 (1980).
K. Nussbaumer. Biopharmazeutische Untersuchungen mit Cyclosporin A: Bioanalytik und Pharmakokinetik. Ph.D. dissertation, University of Basel, Switzerland, 1984.
M. Rowland and T. Tozer.Clinical Pharmacokinetics, Lea and Febiger, Philadelphia, 1980, p. 45.
T. Beveridge. Pharmacokinetics and metabolism of Cyclosporin A. In D. J. G. White (ed.),Cyclosporin A, Elsevier Biomedical Press, Amsterdam, 1982, pp. 35–44.
R. J. Ptachcinski, R. Venkataraman, G. J. Burckart, S. Yang, and T. E. Starzl. Extraction ratio of cyclosporine in a liver transplant patient with organ rejection.J. Pharm. Sci. 74:901–902 (1985).
W. M. Awni and R. J. Sawchuk. The pharmacokinetics of cyclosporine. I. single dose and constant rate infusion studies in the rabbit.Drug Metab. Dispos. 13:127–132 (1985).
W. M. Awni and R. J. Sawchuck. The pharmacokinetics of cyclosporine. II. blood plasma distribution and binding studies.Drug Metab. Dispos. 13:133–138 (1985).
K. Nooter, F. Schultz, and P. Sonneveld. Evidence for a possible dose-dependent pharmacokinetic of cyclosporin A in the rat.Res. Commun. Chem. Pathol. Pharmacol. 43:407–415 (1984).
S. K. Gupta, B. Legg, L. R. Solomon, R. W. G. Johnson, and M. Rowland: Pharmacokinetics of cyclosporin: influence of rate of constant intravenous infusion in renal transplant patients.Br. J. Clin. Pharmacol. 24:519–526 (1987).
C. T. Ueda, M. Lemaire, G. Gsell, P. Misslin, and K. Nussbaumer. Apparent dose-dependent oral absorption of cyclosporin A in rats.Biopharm. Drug Dispos. 5:141–151 (1984).
J. G. Wagner, A modern view of pharmacokinetics.J. Pharmacokin. Biopharm. 1:363–401 (1973).
P. J. McNamara, W. A. Colburn, and M. Gibaldi. Absorption kinetics of hydroflumethiazide.J. Clin. Pharmacol. 18:190–193 (1978).
P. G. Welling, L. L. Lyons, B. S. R. Elliott, and G. L. Amidon. Pharmacokinetics of alcohol following single low doses to fasted and non fasted subjects.J. Clin. Pharmacol. 17:199–206 (1977).
L. R. Whitfield, P. N. Kaul, and M. L. Clark. Chloropromazine metabolism. IX pharmacokinetics of chloropromazine following oral administration in man.J. Pharmacokin. Biopharm. 6:187–196 (1978).
R. M. J. Ings, J. R. Lawrence, A. McDonald, J. McEwan, A. W. Pidgen, and J. D. Robinson. Glibenclamide pharmacokinetics in healthy volunteers: Evidence for zero-order drug absorption.Proc. Br. Pharmacol. Soc.:264p–265p (1981).
T. W. Guentert, N. H. G. Holford, P. E. Coates, R. A. Upton, and S. Riegelman. Quinidine pharmacokinetics in man: choice of a disposition model and absolute bioavailability studies.J. Pharmacokin. Biopharm. 7:315–330 (1979).
M. Thibonnier, N. H. G. Holford, R. A. Upton, C. D. Blume, and R. L. Williams. Pharmacokinetic-pharmacodynamic analysis of unbound disopyramide directly measured in serial plasma samples in man.J. Pharmacokin. Biopharm. 12:559–573 (1984).
E. Redalieu, K. K. H. Chan, V. Tipnis, S. B. Zak, T. G. Gilleran, W. E. Wagner Jr., and A. R. LeSher. Kinetics of hydrochorothiazide absorption in humans.J. Pharm. Sci. 74:765–767 (1985).
J. R. Malagelada, J. S. Robertson, M. L. Brown, M. Remington, J. A. Duenes, G. M. Thomforde, and P. W. Carryer. Intestinal transit of solid and liquid components of a meal in health.Gastroenterology 87:1255–1263 (1984).
R. Wassef, Z. Cohen, S. Nordgren, and B. Langer. Cyclosporine absorption in intestinal transplantation.Transplantation 39:496–499 (1985).
R. Venkataramanan, T. E. Starzl, S. Yang, G. J. Burckart, R. J. Ptachcinski, B. W. Shaw, S. Iwatsuki, D. H. VanThiel, A. Sanghvi, and H. Seltman. Biliary excretion of cyclosporine in liver transplant patients.Transplant. Proc. 17(1):286–289 (1985).
J. Spenard, G. Sirois, and M. A. Gagnon. The second peak in the serum levels curve after oral administrtion of a slow-release quinidine dosage form: effect of food.Br. J. Clin. Pharmacol. 13:752–754 (1982).
P. G. Welling. Influence of food and diet on gastrointestinal drug absorption: a review.J. Pharmacokin. Biopharm. 5:291–334 (1977).
P. G. Welling. Interactions affecting drug absorption.Clin. Pharmacokin. 9:404–434 (1984).
W. Andrews, S. Iwatsuki, and T. E. Starzl. Letter.Transplantation 39:338 (1985).
P. A. Keown, C. R. Stiller, M. Stawecki, J. McMichael, and W. Howson. Pharmacokinetics and interactions of ciclosporin In R. Schindler (ed.),Ciclosporin in Autoimmune Diseases, Springer Verlag, Berlin, 1985, pp. 39–42.
A. J. Wood and M. Lemaire. Pharmacologic aspects of cyclosporine therapy: Pharmacokinetics.Transplant. Proc. 17(4, Suppl 1):27–32 (1985).
R. J. Ptachcinski, R. Venkataramanan, J. T. Rosenthal, G. J. Burckart, R. J. Taylor, and T. R. Hakala. The effect of food on cyclosporine absorption.Transplantation 40:174–176 (1985).
Author information
Authors and Affiliations
Additional information
This work is part of the doctoral dissertation of Jean-Philippe Reymond.
Partly supported by “Contrat de prestation 84112” between INSERM and Sandoz.
Rights and permissions
About this article
Cite this article
Reymond, JP., Steimer, JL. & Niederberger, W. On the dose dependency of Cyclosporin a absorption and disposition in healthy volunteers. Journal of Pharmacokinetics and Biopharmaceutics 16, 331–353 (1988). https://doi.org/10.1007/BF01062550
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062550