Abstract
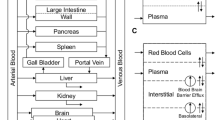
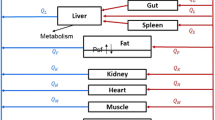
The disposition of the anticancer drug oxantrazole (OX) was characterized in rats bearing the rat glioma-2 (RG-2) brain tumor. Following intraarterial administration of 3 mg/kg of OX, serial sacrifices were completed from 5 min to 5 hr after administration. Blood and tissue samples collected at the time of sacrifice were processed and measured for OX concentrations by HPLC. The kidney had the greatest affinity for OX with the Cmax being 40.6 μg/mlat 15 min after administration. OX concentrations in brain tumor were higher than in normal right and left brain hemispheres, and consistent with enhanced drug blood-tumor barrier (BTB) permeability seen in experimental models for brain tumors. Observed heart, liver, lung, and spleen OX concentrations were similar, ranging from approximately 2 μg/mlto 20 μg/ml. A unique technique was used to develop a global physiological pharmacokinetic model for OX. A hybrid or forcing function method was used to estimate individual tissue compartment biochemical parameters (i.e., partition and mass transfer coefficients). A log likelihood optimization scheme was used to determine the best model structure and parameter sets for each tissue. Most tissues required a 3-subcompartment structure to adequately describe the observed data. The global model was then reconstructed with an arterial blood and rest of body compartments that provided predicted OX concentrations in agreement with the data. The model development strategy provides a systematic approach to physiological pharmacokinetic model development.
Similar content being viewed by others
References
Clinical Brochure of Oxantrazote, NSC 349174. Division of Cancer Treatment, National Cancer Institute, Bethesda, MD, 1987.
S. K. Frank, D. A. Mathiesen, M. Szurszewski. M. J. Kuffel, and M. A. Ames. Preclinical pharmacology of the anthrapyrazole analog oxantrazole (NSC-349174, piroxantrone).Cancer Chemother. Pharmacol. 23:213–218 (1989).
I. R. Judson. Anthrapyrazoles: True successors to the anthracyclines?Anti-Cancer Drugs 2:223–231 (1991).
A. Hantel, R. C. Donehower, E. K. Rowinsky, E. Vance, B. V. Clarke, W. P. McGuirs, D. S. Ettimger, D. A. Noe, and L. B. Grochow. Phase I study and pharmacodynamics of piroxantrone, a new anthrapyrazole.Cancer Res. 50:3284–3288 (1990).
M. M. Ames, C. L. Loprinzi, J. M. Collins, C. Van Haelst-Pisani, R. L. Richardson, J. Rubin, and C. G. Moertel. Phase I clinical pharmacological evaluation of pirozantrone hydrochloride (oxantrazole).Cancer Res. 50:3905–3909 (1990).
N. H. Grieg. Optimizing drug delivery to brain tumors.Cancer Treat. Rev. 14:1–28 (1987).
B. R. Deane and T. L. Lantos. The vasculature of experimental brain tumors, part II. The quantitative assessment of morphological abnormalities.J. Neurol. Sci. 49:67–77 (1981).
N. H. Grieg, H. B. Jones, and J. B. Cavanagh. Blood-brain barrier integrity and host responses in experimental metastatic brain tumors.Clin. Exp. Metastasis 1:229–246, (1983).
R. D. Fross, P. C. Warnke, and D. R. Groothius. Blood flow and blood-to-tissue transport in 9L gliosarcomas: the role of the brain tumor model in drug delivery research.J. Neuro-Oncol. 11:185–197 (1991).
N. H. Grieg. Drug delivery to the brain by blood-brain barrier circumvention and drug modification. In E. A. Neuwelt (ed.),Implications of the Blood-Brain Barrier and Its Manipulation, Vol. 1, Plenum Press, New York, 1989, pp. 311–368.
S. K. Frank, D. A. Mathiesen, L. R. Whitfield, and M. A. Ames. High-performance liquid Chromatographic assay for the experimental anticancer agent oxantrazole.J. Chromatog. 419:225–232 (1987).
E. E. Hassan and J. M. Gallo. High-performance liquid Chromatographic analysis of the anticancer agent oxantrazole in rat blood and tissues.J. Chromatog. 582:225–231 (1992).
E. E. Hassan, R. C. Parish, and J. M. Gallo. Optimized formulation of magnetic chitosan microspheres containing the anticancer agent, oxantrazole.Pharm. Res. 9:390–397 (1992).
E. E. Hassan and J. M. Gallo. Targeting anticancer drugs to the brain: I. Enhanced brain delivery of oxantrazole following administration in magnetic chitosan microspheres.J. Drug Targeting 1:7–14 (1993).
D. R. Groothius, J. M. Fischer, J. F. Pasternak, R. G. Blasberg, N. A. Vick, and D. D. Bigner. Regional measurements of blood-to-tissue transport in experimental RG-2 rat gliomas.Cancer Res. 43:3368–3373 (1983).
M. D. Delp, R. O. Manning, J. V. Bruckner, and R. B. Armstrong. Distribution of cardiac output during diurnal changes of activity in rats.Am. J. Physiol. 261:H1487-H1493 (1991).
K. B. Bischoff and R. G. Brown. Drug distribution in mammals.Chem. Eng. Prog. Symp. 62:33–45 (1966).
H. Nakagawa, D. R. Groothius, E. S. Owens, C. S. Patlak, K. D. Pettigrew, and R. D. Blasberg. Dexamethosone effects on vascular volume and tissue hematocrit in experimental RG-2 gliomas and adjacent brain.J. Neuro-Onco. 6:157–168 (1988).
MINSQ. MicroMath Scientific Software, Salt Lake City, UT, 1989.
SIMUSOLV. Dow Chemical Co., Midland, MI, 1986.
H-S. G. Chen and J. F. Gross. Estimation of tissue-to-plasma partition coefficients used in physiological pharmacokinetic models.J. Pharmacokin. Biopharm. 7:117–125 (1979).
J. M. Gallo, F. C. Lam, and D. G. Perrier. Area method for the estimation of partition coefficients for physiological pharmacokinetic models.J. Pharmacokin. Biopharm. 15:271–280 (1987).
J. M. Gallo, F. C. Lam, and D. G. Perrier. Moment method for the estimation of mass transfer coefficients for physiological pharmacokinetic models.Biopharm. Drug Dispos. 12:127–137 (1991).
F. G. King and R. L. Dedrick. Physiologic model for the pharmacokinetics of 2′-deoxycoformycin in normal and leukemic mice.J. Pharmacokin. Biopharm. 9:519–534 (1981).
J. M. Weissbrod, R. K. Jain, and F. M. Sirotnak. Pharmacokinetics of methotrexate in leukemia cells: Effect of dose and mode of injection.J. Pharmacokin. Biopharm. 6:487–503 (1987).
J. M. Gallo, J. T. Etse, K. Doshi, F. D. Boudinot, and C. K. Chu. Hybrid pharmacokinetic models to describe anti-HIV nucleoside brain disposition following parent and prodrug administration in mice.Pharm. Res. 8:247–253 (1991).
D. Verotta, L. B. Sheiner, W. F. Ebling, and D. R. Stanski. A semiparametric approach to physiological flow models.J. Pharmacokin. Biopharm. 17:463–491 (1989).
Author information
Authors and Affiliations
Additional information
Partial financial support was obtained from DuPont Merck Pharmaceutical Company.
Rights and permissions
About this article
Cite this article
Gallo, J.M., Varkonyi, P., Hassan, E.E. et al. Targeting anticancer drugs to the brain: II. Physiological pharmacokinetic model of oxantrazole following intraarterial administration to rat glioma-2 (RG-2) bearing rats. Journal of Pharmacokinetics and Biopharmaceutics 21, 575–592 (1993). https://doi.org/10.1007/BF01059115
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059115