Abstract
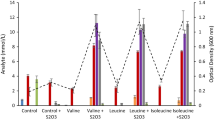
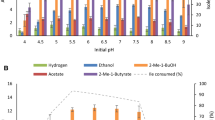
The thermophilicClostridium P2 was isolated from a semi-continuously fed reactor with high ammonium concentration. This bacterium formed substantial amounts of L-alanine as a major fermentation product from glucose, fructose and mannose. Low amounts of acetate, butyrate, carbon dioxide and hydrogen were also formed. A high partial pressure of hydrogen inhibited the degradation of the monosaccharides, whereas hydrogen removal, in the form of methanogenesis was found to be stimulatory. However, the amount of alanine produced per mole of hexose degraded did not change. Hexose degradation and alanine production were favoured by high ammonium concentrations. Nuclear magnetic resonance spectroscopy studies provided strong evidence that an active Embden-Meyerhof-Parnas pathway existed and that alanine was produced via an amination of pyruvate.
Similar content being viewed by others
References
Allcock ER & Woods DR (1982) Carboxymethyl cellulase and cellobiase production byClostridium acetobutylicum in an industrial fermentation medium. Appl. Environ. Microbiol. 41: 539–531
Andreesen JR, Bahl H & Gottschalk G (1989) Introduction to the physiology and biochemistry of the genusClostridium. In: Minton NP & Clarke DJ (Eds) Clostridia. Plenum Press, New York
Blake JS, Salter DN & Smith RH (1981) Synthesis of alanine from ammonia by rumen bacteria. Proc. Nutr. Soc. 40: 4A
Buurman ET, Pennock J, Tempest DW, Joost Teixeira de Mattos M & Neissel OM (1990). Replacement of potassium ions by ammonium ions in different microorganisms grown on potassium limited chemostate culture. Arch. Microbiol. 152: 58–63
Edwards R, Gilroy FV, Jimenez MB & O'Sullivan WJ (1989) Alanine as a major end product of metabolism byGiardia lambia: a proton nuclear magnetic resonance study. Mol. Biochem. Parasitol. 37: 19–26
Erfle JD, Sauer FD & Mahadevan S (1977) Effect of ammonia concentration on activity of enzymes of ammonia assimilation and on synthesis of amino acids by mixed rumen bacteria in continuous culture. J. Dairy. Sci. 60: 1064–1072
Erikson LE (1980) Biomass elemental composition and energy content. Biotechnol. Bioeng. 22: 451–456
Hobson PN & Wallace RJ (1982) Microbial ecology and activities in the rumen. Brit. Rev. Microbiol. 4: 131–191
Houwen FP, Dijkema C, Schoenmakers CHH, Stams AJM & Zehnder AJB (1987)13C-NMR study of propionate degradation by a methanogenic coculture. FEMS Microbiol. Lett. 41: 269–274
Jungerman K, Thauer RK, Leimenstoll G & Decker K (1973) Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim. Biophys. Acta. 305: 268–280
Kengen SWM & Stams AJM (1994) Formation of L-alanine as a reduced end product in carbohydrate fermentation by hyperthermophilic archaeonPyrococcus furiosus. Arch. Microbiol. 161: 168–175
Kengen SWM, de Bok FAM, van Loo N-D, Dijkema C, Stams AJM & de Vos WM (1994) Evidence for the operation of a novel Embden-Meyerhof Pathway that involves ADP-dependent kinases during sugar fermentation byPyrococcus furiosus. J. Biol. Chem. 269: 17537–17541
Örlygsson J, Houwen FP & Svensson BH (1993) Anaerobic degradation of protein and and the role of methane formation in steady state thermophilic enrichment cultures. Swedish J. agric. Res. 23: 45–54
Örlygsson J (1994) The role of interspecies hydrogen transfer on thermophilic protein and amino acid metabolism. PhD Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden
Örlygsson J, Houwen FP & Svensson BH (1995) Thermophilic anaerobic amino acids degradation: deamination rates and end product formation. Appl. Microbiol. Biotechnol. 43: 235–241
Paget TA, Rayner MH, Shipp DWE & Lloyd D (1990)Giardia lamblia produced alanine anaerobically but not in the presence of oxygen. Mol. Biochem. Parasitol. 42: 63–68
Schäfer T & Schönheit P (1992) Maltose fermentation to acetate, C02 and H2 in the anaerobic hyperthemophilic archaeonPyrococcus furiosus: evidence for the operation of a novel sugar fermentation pathway. Arch. Microbiol. 158: 188–202
Sprott GD, Shaw KM & Jarrel KF (1984) Ammonium/potassium exchange in methanogenic bacteria. J. Biol. Chem. 259: 12602–12608
Sprott GD, Shaw KM & Jarrel KF (1985) Methanogens and the K+ transport system are activated by divalent cations in ammoniatreated cells ofMethanospirillum hungatei. J. Biol. Chem. 260: 9244–9250
Sprott GD & Patel GB (1986) Ammonia toxicity in pure cultures of methanogenic bacteria. Syst. Appl. Microbiol. 7: 358–363
Stams AJM & Zehnder AJB (1989) Ecological impact of syntrophic alcohol and fatty acid oxidation. In: Bélaich J-P, Bruschi M & Garcia J-L (Eds) Microbiology and Biochemistry of Strict anaeerobes involved in interspecies hydrogen transfer (pp 87–98). Plenum Press, New York
Thauer RK, Jungermann K & Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41: 100–180
Uhlenbusch I, Sahm H, & Sprenger GA (1991) Expression of an Lalanine dehydrogenase gene inZymomonas mobilis and excretion of L-alanine. Appl. Environ. Microbiol. 57: 1360–1366
Wallace RJ (1979) Effect of ammonia concentration on the composition, hydrolytic activity, and nitrogen metabolism of the microbial flora of the rumen. J. Appl. Bacteriol. 47: 443–455
Weimer PJ & Zcikus JG (1977) Fermentation of cellulose and cellobiose byClostridium thermocellum in the absence and presences ofMethanobacterium thermoautotrophicum. Appl. Environ. Microbiol. 33: 289–297
Wiegant WM (1986) Thermophilic anaerobic degestion of waste and wastewater treatment. PhD Thesis, University of Wageningen, The Netherlands
Wiegel J, Ljungdahl LG & Rawson JR (1979) Isolation of soil and properties of the extreme thermophileClostridium thermohydrosulfuricum. J. Bacteriol. 139: 800–810
Woods DR (1993) The Clostridia and Biotechnology, Butterworth-Heinemann, Boston
Wright DE & Hungate RE (1967) Amino acid concentrations in rumen fluid. Appl. Microbiol. 15: 148–151
Zehnder AJB, Huser BA, Brock TD & Wuhrman K (1980) Characerization of an acetate decarboxylating, non-hydrogen oxidizing methane bacterium. Arch. Microbiol. 124: 1–11
Zhilina TN & Ilarionov SA (1984) Isolation and comparative characteristics of methanogenic bacteria assimilating formate with the description ofMethanobacterium thermoformicicum sp. nov. Microbiologya. 53: 313–321
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Örlygsson, J., Anderson, R. & Svensson, B.H. Alanine as an end product during fermentation of monosaccharides byClostridium strain P2. Antonie van Leeuwenhoek 68, 273–280 (1995). https://doi.org/10.1007/BF00874136
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00874136