Abstract
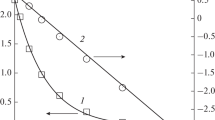
By comparison of the hydrodenitrogenation rate of amylamine with those of neopentylamine and tert-amylamine over a conventional catalyst, it was evidenced that the hydrogen atoms of the carbon in the β position with respect to the nitrogen atom participate in the C-N bond cleavage. A detailed mechanism including the interaction with the catalyst is proposed for this reaction.
Similar content being viewed by others
References
N. Nelson and R.B. Levy, J. Catal. 58 (1979) 485.
R.M. Laine, Catal. Rev.-Sci. Eng. 25 (1983) 459.
Symposium on chemistry of transition metal Sulfides in heterogeneous catalysis of the Division of Petroleum Chemistry of the American Chemical Society, Boston, April 22–27, 1990.
G. Perot, S. Brunei and N. Hamze,Proc. 9th Int. Congress on Catalysis, Calgary 1988, eds. M.J. Phillips and M. Ternan, Vol. 1 (1988) p. 19.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Portefaix, J.L., Cattenot, M., Guerriche, M. et al. Mechanism of carbon-nitrogen bond cleavage during amylamine hydrodenitrogenation over a sulphided NiMo/Al2O3 catalyst. Catal Lett 9, 127–132 (1991). https://doi.org/10.1007/BF00769091
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00769091