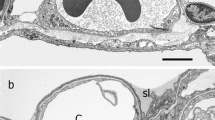
Abstract
Membrane vesicles of physiological as well as inverted orientation can be isolated from mitochrondria. The presence of these vesicles in a membrane can be determined and quantitated by determining the differences between the two vesicle types in terms of rates of NADH oxidation, rates of oxidation of tricarboxylate cycle intermediates, rates of ATP hydrolysis and sensitivity to inhibitors, stimulation of respiration by exogenous cytochromec, inhibition of respiration by polycationic proteins, and visualization of the ATPase by electron microscopy. Procedures to isolate the two membrane types and characteristics of homogeneously oriented preparations are described. Differences in data obtained with homogeneous vesicle preparations and with vesicles of mixed orientation are illustrated. Nonhomogeneously oriented preparations can be enriched in the desired vesicular type by the use of immunoprecipitation, affinity chromatography, and differential centrifugation. The concept of a hybrid vesicle containing oppositely oriented regions is not supported by experimental data.
Similar content being viewed by others
References
Albracht, S. P. J., and Heidrich, H.-G. (1975).Biochim. Biophys. Acta 376, 231–236.
Astle, L., and Cooper, C. (1974).Biochemistry 13, 154–160.
Bentzel, C. J., and Solomon, A. K. (1967).J. Gen. Physiol. 50, 1547–1563.
Berry, E. A., and Hinkle, P. C. (1983).J. Biol. Chem. 258, 1474–1486.
Branca, D., Ferguson, S. J., and Sorgato, M. C. (1981).Eur. J. Biochem. 116, 341–346.
Chance, B., and Hagihara, B. (1963).Proc. Fifth Int. Congr. Biochem. 5, 3–33.
Chance, B., Erecinska, M., and Lee, C. P. (1970).Proc. Natl. Acad. Sci. USA 66, 928–935.
Crane, F. L., Glenn, J. L., and Green, D. E. (1956).Biochim. Biophys. Acta 22, 475–487.
DePierre, J. W., and Ernster, L. (1977).Annu. Rev. Biochem. 46, 201–262.
Donnellan, J. F., Barker, M. D., Wood, J., and Beechey, R. B. (1970).Biochem. J. 120, 467–478.
D'Souza, M. P., and Lindsay, J. G. (1981).Biochim. Biophys. Acta 640, 463–472.
Eytan, G. D., Carroll, R. C., Schatz, G., and Racker, E. (1975).J. Biol. Chem. 250, 8598–8603.
Fleischer, S., Meissner, G., Smigel, M., and Wood, R. (1974).Methods Enzymol. 31, 292–299.
Gautheron, D. C., Godinot, C., Mairouch, H., Blanchy, B., and Wojtkowiak, Z. (1977). InBioenergetics of Membranes (Packer, L., Papageorgiou, G. C., and Trebst, A., eds.), Elsevier, Amsterdam, pp. 501–512.
Godinot, C., and Gautheron, D. C. (1979).Methods Enzymol. 55, 112–114.
Hackenbrock, C. R., and Hammon, K. M. (1975).J. Biol. Chem. 250, 9185–9197.
Hansen, M., and Smith, A. L. (1964).Biochim. Biophys. Acta 81, 214–222.
Hare, J. F., Olden, K., and Kennedy, E. P. (1974).Proc. Natl. Acad. Sci. USA 71, 4843–4846.
Hare, J. F., and Crane, F. L. (1971).J. Bioenerg. 2, 317–326.
Harmon, H. J. (1982).J. Bioenerg. Biomembr. 14, 377–386.
Harmon, H. J., and Crane, F. L. (1974).Biochem. Biophys. Res. Commun. 59, 326–333.
Harmon, H. J., and Crane, F. L. (1976).Biochem. Biophys. Acta 440, 45–58.
Harmon, H. J., and Basile, P. F. (1982).J. Bioenerg. Biomembr. 14, 23–43.
Harmon, H. J., and Sanborn, M. R. (1982).Env. Res. 29, 160–173.
Harmon, H. J., Hall, J. D., and Crane, F. L. (1974).Biochim. Biophys. Acta 344, 119–155.
Harris, E. J., and van Dam, K. (1968).Biochem. J. 106, 759–766.
Hatefi, Y., and Lester, R. L. (1958).Biochim. Biophys. Acta 27, 83–88.
Hinkle, P. C., and Horstman, L. L. (1971).J. Biol. Chem. 246, 6024–6028.
Horstman, L. L., and Racker, E. (1970).J. Biol. Chem. 245, 1336–1344.
Huang, C. H., and Lee, C. P. (1975).Biochim. Biophys. Acta 376, 398–414.
Huang, C. H., Keyhani, E., and Lee, C. P. (1973).Biochim. Biophys. Acta 305, 455–473.
Husain, I., and Harris, D. A. (1983).FEBS Lett. 160, 110–114.
Jacobs, E. E., and Sanadi, D. R. (1960).J. Biol. Chem. 235, 531–534.
Kagawa, Y., and Racker, E. (1966).J. Biol. Chem. 241, 2475–2482.
Keilin, D., and Hartree, E. F. (1940).Proc. R. Soc. London, Ser. B 129, 277–306.
Klingenberg, M. (1981). InMitochondria and Microsomes (Lee, C. P., Schatz, G., and Pallner, G., eds.), Addison-Wesley, Reading, Massachusetts, pp. 293–316.
Klingenberg, M., and Bucholz, M. (1970).Eur. J. Biochem. 13, 246–252.
Lee, C. P. (1971). InProbes of Structure and Function of Macromolecules and Membranes, Vol. I. Probes and Membrane Function (Chance, B., Lee, C. P., and Blaise, J. K., eds.), Academic Press, New York, pp. 417–426.
Lee, C. P. (1979).Methods Enzymol. 55, 105–112.
Lehninger, A. L. (1955). The Harvey Lectures 1953–1954, Academic Press, New York, pp. 176–215.
Lenaz, G., and MacLennan, D. H. (1966).J. Biol. Chem. 241, 5260–5265.
Lotscher, H. R., Schwerzmann, K., and Carafoli, E. (1979).FEBS Lett. 99, 194–198.
Mackler, B., and Green, D. E. (1956).Biochim. Biophys. Acta 21, 1–6.
Malviya, A. N., Parsa, B., Yodaiken, R. E., and Elliott, W. B. (1968).Biochim. Biophys. Acta 162, 195–209.
McIntyre, J. O., Bock, H.-G. O., and Fleischer, S., (1978).Biochim. Biophys. Acta 513, 255–267.
Mitchell, P., and Moyle, J. (1967).Biochem. J. 104, 588–600.
Mitchell, P., and Moyle, J. (1969).Eur. J. Biochem. 7, 471–484.
Moore, A. L., and Bonner, W. D., Jr. (1981).Biochim. Biophys. Acta 634, 117–128.
Moury, D. N., and Crane, F. L. (1964).Biochem. Biophys. Res. Commun. 15, 442–446.
Muscatello, V., and Carafoli, E. (1969).J. Cell. Biol. 40, 602–621.
Nedergaard, J., and Cannon, B. (1979).Methods Enzymol. 55, 3–28.
Papa, S., Storey, B. T., Lorusso, M., Lee, C. P., and Chance, B. (1973a).Biochem. Biophys. Res. Commun. 52, 1395–1402.
Papa, S., Guerrieri, F., Simone, S., Lorusso, M., and Larosa, D. (1973b).Biochim. Biophys. Acta 292, 20–38.
Quintanilha, A. T., and Packer, L. (1977).Proc. Natl. Acad. Sci. USA 74, 570–574.
Racker, E., and Horstman, L. L. (1967).J. Biol. Chem. 242, 2547–2551.
Rasmussen, U. F. (1969).FEBS Lett. 2, 157–162.
Reynafarje, B., Brand, M. D., Alexandre, A., and Lehninger, A. L. (1979).Methods Enzymol. 55, 640–656.
Robinson, J. B., and Srere, P. A. (1985).J. Biol. Chem. 260, 10800–10805.
Rosier, R. N., Gunter, T. E., and Gunter, K. K. (1980).Fed. Proc. 39, 2057.
Ruzicka, F. J., and Crane, F. L. (1971).Biochim. Biophys. Acta 226, 221–233.
Scholte, H. R., Weijers, P. J., and Wit-Peeters, E. M. (1973).Biochim. Biophys. Acta 291, 764–773.
Schwartz, A. (1974).J. Biol. Chem. 240, 939–943.
Schwerzmann, K., and Pedersen, P. L. (1981).Biochemistry 20, 6305–6311.
Smith, A. L. (1967).Methods Enzymol. 10, 81–86.
Smith, L., and Conrad, H. (1956).Arch. Biochem. Biophys. 63, 403–413.
Smith, L., and Minnaert, K. (1965).Biochim. Biophys. Acta 105, 1–14.
Smith, L., Davies, H. C., and Nava, M. E. (1980).Biochem. J. 19, 4261–4265.
Smith, S., and Ragan, C. I. (1980).Biochem. J. 185, 315–326.
Storey, B. T., Scott, D. M., and Lee, C. P. (1980).J. Biol. Chem. 255, 5224–5229.
Thayer, W. S., and Rubin, E. (1979).J. Biol. Chem. 254, 7717–7723.
Tsou, C. L. (1952).Biochem. J. 50, 493–499.
Vercesi, A., Reynafarje, B., and Lehninger, A. L. (1978).J. Biol. Chem. 253, 6379–6385.
Vinogradov, A. D., and King, T. E. (1979).Methods Enzymol. 55, 118–127.
Wehrle, J. P., Cintron, N. M., and Pedersen, P. L. (1978).J. Biol. Chem. 253, 8598–8603.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harmon, H.J. Determination of the orientation of membrane vesicles derived from mitochondria. J Bioenerg Biomembr 19, 167–189 (1987). https://doi.org/10.1007/BF00762723
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00762723