Summary
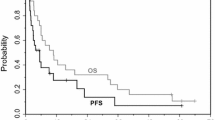
Carboplatin (400 mg/m2) was given at 28-day intervals to 41 patients with malignant mesothelioma. In all, 40 patients were eligible and evaluable for response. Partial responses were seen in 2 cases (5%); regression of evaluable disease, in 1 patient (2%); and stable disease, in 19 subjects (48%). A median of two doses of carboplatin per patient resulted in mild toxicity. Leukopenia (≤2,000 cells/μl) and thrombocytopenia (<100,000 cells/μl) were seen in only 6% and 20% of the patients, respectively. Median survival from study entry was estimated at 7.1 months, with a 1-year survival of 25%±7%. Carboplatin given at a dose of 400 mg/m2 at 28-day intervals shows minor activity against malignant mesothelioma.
Similar content being viewed by others
References
Aisner J, Sigman LM (1987) The role of chemotherapy in the treatment of malignant mesothelioma. In: Antman K, Aisner J (eds) Asbestos related malignancy. Grune & Stratton, Philadelphia, p 385
Alberts AS, Falkson G, Goedhals L, Vorobiof DA, Van Der Merwe CA (1988) Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol 6: 527
Antman K, Shemin R, Ryan L, et al. (1988) Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965–1985. J Clin Oncol 6: 147
Antman KH, Li FP, Osteen R, et al. (1989) Mesothelioma. In: DeVita VT Jr, Hellman S, Rosenberg SA (eds) Updates to Cancer: principles and practice of oncology, 3rd edn, vol 3 (1). J. B. Lippincott, Philadelphia
Atkinson NE, Brown BW (1985) Confidence limits for probability of response in multistage phase II clinical trials. Biometrics 41: 741
Bajorin D, Kelson D, Mintzer DM (1987) Phase II trial of mitomycin in malignant mesothelioma. Cancer Treat Rep 71: 857
Calvert AH, Newell DR, Gumbrell LA (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748
Cantwell BM, Franks CR, Harris AL (1986) A phase II study of the platinum analogues JM8 and JM9 in malignant pleural mesothelioma. Cancer Chemother Pharmacol 18: 286
Chahinian AP, Szrajer L, Malamud S, Schwartz PH, Holland JH (1985) Comparative activity of three platinum analogues against human malignant mesothelioma (HMM) xenografts in nude mice. Proc Am Assos Cancer Res 26: 261
Chahinian AP, Antman K, Aisner J (1987) Cisplatin with adriamycin or mitomycin for malignant mesothelioma: A randomized phase II trial. Proc Am Soc Clin Oncol 6: 183
Djerassi I, Kim JS, Kassarov L, Reggev A (1985) Response of mesothelioma to large doses of methotrexate with CF rescue (HDMTX-CF) used alone or with cis-platinum. Proc Am Soc Clin Oncol 4: 191
Egorin MJ, Van Echo DA, Tipping SJ, et al. (1984) Pharmacokinetics and dose reduction ofcis-diammine-(1,1-cyclobutanedicarboxylato) platinum in patients with impaired renal function. Cancer Res 44: 5432
Gore ME, Calvert AH, Smith IE (1987) High dose carboplatin in the treatment of lung cancer and mesothelioma: A phase I dose escalation study. Eur J Cancer Clin Oncol 23: 1391
Hsu S, Hsu S, Zhao X, Koo-Shan CS, Whang-Peng J (1988) Establishment of human mesothelioma cell lines (MS-1,-2) and production of a monoclonal antibody (Anti MS) with diagnostic and therapeutic potential. Cancer Res 48: 5228
Mbidde EK, Harland SJ, Calvert AH, Smith IE (1986) Phase II trial of carboplatin (JM8) in treatment of patients with malignant mesothelioma. Cancer Chemother Pharmacol 18: 284
Mintzer DM, Kelson D, Frimmer D, Heeleen P, Gralla R, Ochoa M (1985) Phase II trial of high-dose cisplatin in patients with malignant mesothelioma. Cancer Treat Rep 69: 711
Popescu NC, Amsbaugh SC, Chahinian AP, Di Paola JS (1987) Non-random deletions of chromosome 3 (P14–21) in human malignant mesothelioma. Proc Am Assoc Cancer Res 28: 27
Raghavan D, Gianoutsos P, Bishop J, Lee J, Young I, Corte P, Bye P, McCaughan B (1989) Phase II trial of carboplatin in the management of malignant mesothelioma. J Clin Oncol 8: 151
Shea TC, Flaherty M, Elias A, et al. (1989) A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol 7: 651
Vogelzang NJ, Schultz SM, Iannucci AM, Kennedy BJ (1984) Malignant mesothelioma: The University of Minnesota experience. Cancer 53: 377
Wolf KM, Piotrowski ZH, Engel JD, Bekeris LG, Palacios E, Fisher KA (1987) Malignant mesothelioma with occupational and environmental asbestos exposure in an Illinois Community Hospital. Arch Int Med 147: 2145
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vogelzang, N.J., Goutsou, M., Corson, J.M. et al. Carboplatin in malignant mesothelioma: A phase II study of the cancer and leukemia Group B. Cancer Chemother. Pharmacol. 27, 239–242 (1990). https://doi.org/10.1007/BF00685720
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685720