Abstract
-
1.
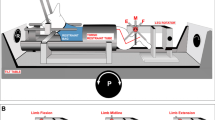
The activity of presumably inhibitory reticulospinal neurons with cell bodies located in the medial aspects of the medullary reticular formation and axons projecting to lumbosacral cord has been recorded in decerebrate cats and their response characteristics to sinusoidal stimulation of labyrinth receptors (134 neurons) and neck receptors (110 neurons) have been related to cell size inferred from the conduction velocity of the corresponding axons.
-
2.
No significant correlation was found between resting discharge and conduction velocity of the axons.
-
3.
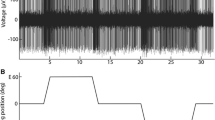
Among the recorded reticulospinal neurons, 64/134 (i.e. 47.8%) units responded to roll tilt, while 66/110 (i.e. 60.0%) units responded to neck rotation (0.026 Hz, ±10°). A positive correlation was found between gain (imp./s/deg) of the labyrinth and neck responses and conduction velocity of the axons. Thus, due to absence of correlation between resting discharge and conduction velocity of the axons, larger neurons exhibited a greater percentage modulation (sensitivity) to the labyrinth and the neck input than smaller neurons. These findings are attributed to an overall increase in density or efficacy of the synaptic contacts made by the vestibular and neck afferent pathways on reticulospinal neurons of increasing size.
-
4.
Units receiving neck-macular vestibular convergence showed on the average an higher gain of the neck (G N) response with respect to the labyrinth (G L) response (G N/G:L: 1.95±1.49, S.D.;n=43); however, due to a parallel increase in gain of the reticulospinal neurons to both neck and labyrinth inputs, the relative effectiveness of the two inputs did not vary in different units as a function of cell size.
-
5.
The reticulospinal neurons were mainly excited by the direction of animal orientation and/or neck displacement. In particular, most of these positional sensitive units were excited by side-up animal tilt (37/58, i.e. 63.8%) and by sidedown neck rotation (47/60, i.e. 78.3%). These predominant response patterns were particularly found between large size neurons, whereas small size neurons tended to show also other response patterns.
-
6.
The evidence indicates that in addition to intrinsic neuronal properties related to cell size, the quantitative and qualitative organization of synaptic inputs represents the critical factor controlling the responsiveness of reticulospinal neurons to vestibular and neck stimulation.
Similar content being viewed by others
References
Anderson JH, Blancks RHI, Precht W (1978) Response characteristics of semicircular canal and otolith systems in cat. I. Dynamic response of primary vestibular fibers. Exp Brain Res 32:491–507
Barrett JN, Crill WE (1971) Specific membrane resistance resistivity of dye-injected cat motoneurons. Brain Res 28:556–561
Berman AL (1968) The brain stem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin Press, Madison, Wisconsin
Bizzi E, Pompeiano O, Somogyi J (1964) Spontaneous activity of single vestibular neurons of unrestrained cats during sleep and wakefulness. Arch Ital Biol 102:308–330
Bodian D (1946) Spinal projections of brainstem in rhesus monkey deduced from retrograde chromatolysis. Anat Rec 94:512–513
Boyle R, Pompeiano O (1980a) Reciprocal responses to sinusoidal tilt of neurons in Deiters' nucleus and their dynamic characteristics. Arch Ital Biol 118:1–32
Boyle R, Pompeiano O (1980b) Responses of vestibulospinal neurons to sinusoidal rotation of neck. J Neurophysiol 44:633–649
Boyle R, Pompeiano O (1981a) Convergence and interaction of neck and macular vestibular inputs on vestibulospinal neurons. J Neurophysiol 45:852–868
Boyle R, Pompeiano O (1981b) Relation between cell size and response characteristics of vestibulospinal neurons to labyrinth and neck inputs. J Neuroscience 1:1052–1066
Boyle R, Pompeiano O (1981c) Response of vestibulospinal neurons to neck and macular vestibular inputs in the presence or absence of the paleocerebellum. Ann NY Acad Sci 374:373–394
Brodal A (1957) The reticular formation of the brain stem. Oliver and Boyd, Edinburgh
Burke RE (1973) On the central nervous system control of fast and slow twitch motor units. In: Desmedt JE (ed) New developments in electromyography and clinical neurophysiology, vol 3. Karger, Basel, pp 69–94
Burke RE (1979) The role of synaptic organization in the control of motor unit activity during movement. In: Granit R, Pompeiano O (eds) Progress in brain research. Reflex control of posture and movement, vol 50. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 61–67
Burton H, Loewy AD (1977) Projections to the spinal cord from medullary somatosensory relay nuclei. J Comp Neurol 173:773–792
Coulter JD, Bowker RM, Wise SP, Murray EA, Castiglioni AJ, Westlund KN (1979) Cortical, tectal and medullary descending pathways to the cervical spinal cord. In: Granit R, Pompeiano O (eds) Progress in brain research. Reflex control of posture and movement, vol 50. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 263–279
Cullheim S (1978) Relations between cell body size, axon diameter and axon conduction velocity of cat sciatic α-motoneurons stained with horseradish peroxidase. Neurosci Lett 8:17–20
Denoth F, Magherini PC, Pompeiano O, Stanojević M (1979) Responses of Purkinje cells of the cerebellar vermis to neck and macular vestibular inputs. Pflügers Arch 381:87–98
Denoth F, Magherini PC, Pompeiano O, Stanojević M (1980) Responses of Purkinje cells of cerebellar vermis to sinusoidal rotation of neck. J Neurophysiol 43:46–59
Eccles JC, Nicoll RA, Schwarz DWF, Tábořiková H, Willey TJ (1975) Reticulospinal neurons with and without monosynaptic inputs from cerebellar nuclei. J Neurophysiol 38:513–530
Hayes NL, Rustioni A (1981) Descending projections from brainstem and sensorimotor cortex to spinal enlargements in the cat. Single and double retrograde tracer studies. Exp Brain Res 41:89–107
Hellon RF (1971) The marking of electrode tip positions in nervous tissue. J Physiol (Lond) 214:12P
Henneman E, Mendell LM (1981) Functional organization of motoneuron pool and its inputs. Handbook of physiology. The nervous system. II. Part 1. American Physiological Society, Bethesda, Maryland, pp 423–507
Ito M, Udo M, Mano N (1970) Long inhibitory and excitatory pathways converging onto cat reticular and Deiters' neurons and their relevance to reticulofugal axons. J Neurophysiol 33:210–226
Jankowska E, Lund S, Lundberg A, Pompeiano O (1968) Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol 106:124–140
Kasper H, Thoden U (1981) Effects of natural neck afferent stimulation on vestibulo-spinal neurons in the decerebrate cat. Exp Brain Res 44:401–408
Kernell D (1966) Input resistance, electrical excitability and size of ventral horn cells in cat spinal cord. Science 152:1637–1640
Kernell D, Zwaagstra B (1981) Input conductance, axonal conduction velocity and cell size among hindlimb motoneurones of the cat. Brain Res 204:311–326
Kubin L, Manzoni D, Pompeiano O (1981) Responses of lateral reticular neurons to convergent neck and macular vestibular inputs. J Neurophysiol 46:48–64
Kuypers HGJM, Maisky VA (1975) Retrograde axonal transport of horseradish peroxidase from spinal cord to brain stem cell groups in the cat. Neurosci Lett 1:9–14
Llinás R, Terzuolo CA (1964) Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha extensor motoneurons. J Neurophysiol 27:579–591
Llinás R, Terzuolo CA (1965) Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons. J Neurophysiol 28:413–422
Lund S, Pompeiano O (1968) Monosynaptic excitation on alphamotoneurones from supraspinal structures in the cat. Acta Physiol Scand 73:1–21
Magni F, Willis WD (1963) Identification of reticular formation neurons by intracellular recording. Arch Ital Biol 101:681–702
Magoun HW, Rhines R (1946) An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol 9:165–171
Magoun HW, Rhines R (1947) Spasticity. The stretch-reflex and extrapyramidal systems. Charles C. Thomas, Springfield, Ill.
Manzoni D, Pompeiano O, Stampacchia G, Srivastava UC (1983) Responses of medullary reticulospinal neurons to sinusoidal stimulation of labyrinth receptors in decerebrate cat. J Neurophysiol (in press)
Peterson BW, Maunz RA, Pitts NG, Mackel R (1975) Patterns of projection and branching of reticulospinal neurons. Exp Brain Res 23:333–351
Peterson BW, Pitts NG, Fukushima K (1979) Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36:1–20
Pilyavsky AI, Gokin AP (1978) Investigation of the cortico-reticulospinal connections in cats. Neuroscience 3:99–103
Pompeiano O (1975) Vestibulo-spinal relationships. In: Naunton RF (ed) The vestibular system. Academic, New York, pp 147–180
Pompeiano O, Manzoni D, Srivastava UC, Stampacchia G (1983a) Convergence and interaction of neck and macular vestibular inputs on reticulospinal neurons. Neuroscience (submitted)
Pompeiano O, Xerri C, Gianni S, Manzoni D (1983b) Central compensation of vestibular deficits. II. Influences of roll tilt on different size lateral vestibular neurons after ipsilateral labyrinth deafferentation. J Neurophysiol (submitted)
Precht W (1974) The physiology of the vestibular nuclei. In: Kornhuber HH (ed) Handbook of sensory physiology, vol VI/1. Vestibular system. Part 1. Basic mechanisms. Springer, Berlin Heidelberg New York, pp 353–416
Schor RH, Miller AD (1982) Relationship of cat vestibular neurons to otolith-spinal reflexes. Exp Brain Res 47:137–144
Shimazu H, Precht W (1965) Tonic and kinetic responses of cat's vestibular neurons to horizontal angular acceleration. J Neurophysiol 28:991–1013
Srivastava UC, Manzoni D, Pompeiano O, Stampacchia G (1983) Responses of medullary reticulospinal neurons to sinusoidal rotation of neck in the decrerebrate cat. Neuroscience (in press)
Strauss P, Saling M, Pilyavski AI, Pavlasek J, Hlavacka F (1982) Electrophysiological characteristics of reticulospinal neurones in relation to the conduction velocity of their axones. Physiol Bohemoslov 31:101–112
Tohyama M, Sakai K, Salvert D, Jouvet M (1979) Spinal projections from the lower brain-stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origin of the reticulospinal tracts and their funicular trajectories. Brain Res 173:383–403
Torvik A, Brodal A (1957) The origin of reticulospinal fibers in the cat. An experimental study. Anat Rec 128:113–138
Wilson VJ, Melvill Jones G (1979) Mammalian vestibular physiology. Plenum Press, New York
Wolstencroft VH (1964) Reticulospinal neurones. J Physiol (Lond) 174:91–108
Zemlan FP, Pfaff DW (1979) Topographical organization in medullary reticulospinal systems as demonstrated by the horseradish peroxidase technique. Brain Res 174:161–166
Zucker RS (1973) Theoretical implications of the size principle of motoneuron recruitment. J Theor Biol 38:587–596
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pompeiano, O., Manzoni, D., Srivastava, U.C. et al. Relation between cell size and response characteristics of medullary reticulospinal neurons to labyrinth and neck inputs. Pflugers Arch. 398, 298–309 (1983). https://doi.org/10.1007/BF00657239
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00657239