Summary
The influence of the red cell concentration of 2,3-diphosphoglycerate (2,3-DPG, 0.5–26 μmoles/g erythrocytes) on the “CO2-Bohr effect” (pH varied by CO2 at constant base excess) and the “fixed acid-Bohr effect” (pH varied by fixed acid or base at constantP CO2) was studied in human blood at plasma pH values ranging between pH 7.2 and pH 7.6.
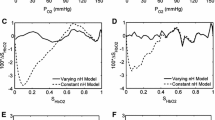
Elevation of red cell 2,3-DPG concentration leads to a numerical decrease of the “CO2-Bohr coefficient” referring to plasma pH. The “fixed acid-Bohr coefficients” are numerically smaller than the corresponding “CO2-Bohr coefficients” and exhibit a maximum at normal red cell 2,3-DPG concentrations. The Bohr coefficients referring to red cell pH are distinctly higher than those referring to plasma pH, especially at high 2,3-DPG levels. This is due on the one hand to the physico-chemical properties of the intact red cell membrane, and on the other hand to a 2,3-DPG-induced decrease in the ratio ΔpHcell/ΔpHplasma.
From the results it is concluded that 2,3-DPG exerts a dual effect on the Bohr coefficients of whole blood which is mediated 1. by the direct effect of 2,3-DPG on the allosteric properties of hemoglobin (as reflected by changes of the Bohr coefficients referring to red cell pH), and 2. by the effect of 2,3-DPG on ΔpHcell/ΔpHplasma.
Similar content being viewed by others
References
Arnone, A.: X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhemoglobin. Nature (Lond.)237, 146–149 (1972)
Arturson, G., Garby, L., Robert, M., Zaar, B.: The oxygen dissociation curve of normal human blood with special reference to the influence of physiological effector ligands. Scand. J. clin. Lab. Invest.34, 9–14 (1974)
Arturson, G., Garby, L., Wranne, B., Zaar, B.: Effect of 2,3-diphosphoglycerate on the oxygen affinity and on the proton- and carbamino-linked oxygen affinity of hemoglobin in human whole blood. Acta physiol. scand.92, 332–340 (1974)
Bartels, H.: The biological significance of the Bohr effect. In: Oxygen affinity of hemoglobin and red cell acid base status (M. Rørth and P. Astrup, eds.), pp. 717–734. Copenhagen: Munksgaard 1972. New York: Academic Press, 1972
Battaglia, P., Morpurgo, G., Passi, S.: Variability of the Bohr effect in man. Experientia (Basel)27, 321–322 (1971)
Bauer, Ch.: Antagonistic influence of CO2 and 2,3-diphosphoglycerate on the Bohr effect of human hemoglobin. Life Sci.8, 1041–1046 (1969)
Bauer, C.: Reduction of the carbon dioxide affinity of human hemoglobin solutions by 2,3-diphosphoglycerate. Respir. Physiol.10, 10–19 (1970)
Benesch, R. E., Benesch, R., Yu, C. I.: The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength and hemoglobin concentration. Biochemistry8, 2567–2571 (1969)
de Bruin, S. H., Rollema, H. S., Janssen, L. H. M., van Os, A. J.: The interaction of 2,3-diphosphoglycerate with human deoxy- and oxyhemoglobin. Biochem. biophys. Res. Commun.58, 204–209 (1974)
Bursaux, E., Freminet, A., Poyart, C. F.: The Bohr effect, the Donnan equilibrium and the estimation of P50 in human whole blood. Bull. Physiopath. Resp.8, 755–768 (1972)
Caldwell, P. R. B., Nagel, R. L., Jaffe, E. R.: The effect of oxygen, carbon dioxide, pH and cyanate on the binding of 2,3-diphosphoglycerate to human hemoglobin. Biochem. biophys. Res. Commun.44, 1504–1509 (1971)
Deuticke, B., Duhm, J., Dierkesmann, R.: Maximal elevation of 2,3-diphosphoglycerate concentrations in human erythrocytes: Influence on glycolytic metabolism and intracellular pH. Pflügers Arch.326, 15–34 (1971)
Ditzel, J., Standl, E.: The oxygen transport system during diabetic ketoacidosis and recovery. Diabetologia11, 255–260 (1975)
Duhm, J.: Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on the oxygen affinity and intracellular pH of human erythrocytes. Pflügers Arch.326, 341–356 (1971)
Duhm, J.: Studies on 2,3-diphosphoglycerate: Effects on hemoglobin, glycolysis and on buffering properties of human erythrocytes. In: Erythrocyte structure and function, vol. 1, Progress in clinical and biological research (G. Brewer, ed.), pp. 167–197. New York: Alan R. Liss 1975
Duhm, J.: Glycolysis in human erythrocytes containining elevated concentrations of 2,3-P2-glycerate. Biochim. biophys. Acta (Amst.)385, 68–80 (1975)
Duhm, J.: Influence of 2,3-diphosphoglycerate on the buffering properties of human blood. Role of the red cell membrane. Pflügers Arch.363, 61–67 (1976)
Duhm, J., Gerlach, E.: Metabolism and function of 2,3-diphosphoglycerate in red blood cells. In: The human red cell in vitro (T. Greenwalt and G. A. Jamieson, eds.), pp. 111–148. New York-London: Grune and Stratton 1974
Funder, J., Wieth, J. O.: Chloride and hydrogen ion distribution between human red cells and plasma. Acta physiol. scand.68, 234–245 (1966)
Garby, L., Robert, M., Zaar, B.: Proton- and carbaminolinked oxygen affinity of normal human blood. Acta physiol. scand.84, 482–492 (1972)
Gerlach, E., Deuticke, B.: Eine einfache Methode zur Mikrobestimmung von Phosphat in der Papierchromatographie. Biochem. Z.337, 477–479 (1963)
Gerlach, E., Deuticke, B., Duhm, J.: Phosphat-Permeabilität und Phosphat-Stoffwechsel menschlicher Erythrocyten und Möglichkeiten ihrer experimentellen Beeinflussung. Pflügers Arch.280, 243–274 (1964)
Hilpert, P., Fleischmann, R. G., Kempe, D., Bartels, H.: The Bohr effect related to blood and erythrocyte pH. Amer. J. Physiol.205, 337–340 (1963)
Hlastala, M. P., Woodson, R. D.: Saturation dependency of the Bohr effect: Interactions among H+, CO2 and DPG. J. appl. Physiol.38, 1126–1131 (1975)
Kilmartin, J. V.: Influence of DPG on the Bohr effect of human hemoglobin. FEBS Letters38, 147–148 (1974)
Meier, U., Böning, D., Rubenstein, H. J.: Oxygenation dependent variations of the Bohr coefficient related to whole blood and erythrocyte pH. Effect of lactic and carbonic acid. Pflügers Arch.349, 203–213 (1974)
Messier, A. A., Shaefer, K. E.: The Bohr effect in chronic hypercapnia. Respir. Physiol.19, 26–34 (1973)
Morpurgo, G., Battaglia, P., Carter, N. D., Modiano, G., Passi, S.: The Bohr effect and the red cell 2,3-DPG and Hb content in Sherpas and Europeans at low and at high altitude. Experientia (Basel)28, 1280–1283 (1972)
Naeraa, N., Petersen, E. S., Boye, E., Severinghaus, J. W.: pH and molecular CO2 components of the Bohr effect in human blood. Scand. J. clin. Lab. Invest.18, 96–102 (1966)
Proctor, H. J., Parker, J. C.: Treatment of severe hypoxia by transfusion with red cells high in 2,3-diphosphoglycerate (2,3-DPG). Clin. Res.20, 497 (abstract) (1972)
Riggs, A.: The nature and significance of the Bohr effect in mammalian hemoglobins. J. gen. Physiol.43, 737–752 (1960)
Riggs, A.: Mechanisms of the enhancement of the Bohr effect in mammalian hemoglobins by diphosphoglycerate. Proc. nat. Acad. Sci. (Wash.)68, 2062–2065 (1971)
Rossi-Bernardi, L., Roughton, F. J. W.: The specific influence of carbon dioxide and carbamate compounds in the buffer power and Bohr effects in human hemoglobin solutions. J. Physiol. (Lond.)189, 1–29 (1967)
Ruckpaul, K., Scheler, W., Jung, F.: Zur Veränderung der häm-gekoppelten Ionisation von Proteingruppen in Hämoglobnen verschiedener Spezies durch eine Reihe von Alkylisozyaniden. Acta biol. med. germ.28, 751–759 (1972)
Salhany, J. M., Keitt, A. S., Eliot, R. S.: The rate of deoxygenation of red blood cells: Effect of intracellular 2,3-diphosphoglycerate and pH. FEBS Letters16, 257–261 (1971)
Severinghaus, J. W.: Blood gas calculator. J. appl. Physiol.21, 1108–1116 (1966)
Shappell, S., Lenfant, C. J. M.: Adaptive, genetic and iatrogenic alterations of the oxyhemoglobin-dissociation curve. Anesthesiology37, 127–139 (1972)
Siggaard-Andersen, O.: The acid-base status of the blood. Copenhagen. Munksgaard 1974
Siggaard-Andersen, O., Garby, L.: The Bohr effect and the Haldane effect. Scand. J. clin. Lab. Invest.31, 1–8 (1973)
Siggaard-Andersen, O., Jørgensen, K., Naeraa, N.: Spectrophotometric determination of oxygen saturation in capillary blood. Scand. J. Lab. clin. Invest.14, 298–302 (1962)
Siggaard-Andersen, O., Rørth, M., Nörgaard-Pedersen, B., Sparre-Andersen, O., Johansen, E.: Oxygen-linked hydrogen binding of human hemoglobin. Effects of carbon dioxide and 2,3-diphosphoglycerate. IV. Thermodynamic relationship between the variables. Scand. J. clin. Lab. Invest.29, 303–320 (1972)
Siggaard-Andersen, O., Salling, N.: Oxygen-linked hydrogen ion binding of human hemoglobin. Effects of carbon dioxide and 2,3-diphosphoglycerate. II. Studies on whole blood. Scand. J. clin. Lab. Invest.27, 361–366 (1971)
Siggaard-Andersen, O., Salling, N., Nörgaard-Pedersen, B., Rørth, M.: Oxygen-linked hydrogen ion binding of human hemoglobin. Effects of carbon dioxide and 2,3-diphosphoglycerate. III. Comparison of the Bohr effect and the Haldane effect. Scand. J. clin. Lab. Invest.29, 185–193 (1972)
Suwa, K., Bendixen, H. H.: The Bohr factor: Is it constant? Fed. Proc.31, 355 (abstract) (1972)
Tomita, S., Riggs, A.: Studies of the interaction of 2,3-diphosphoglycerate and carbon dioxide with hemoglobins from mouse, man and elephant. J. biol. Chem.246, 547–554 (1971)
Wood, S. C., Johansen, K.: Adaptation to hypoxia by increased HbO2 affinity and decreased red cell ATP concentration. Nature New Biol.237, 278–279 (1972)
Wranne, B., Woodson, R. D., Detter, J. C.: Bohr effect: Interaction between H+, CO2 and 2,3-DPG in fresh and stored blood. J. appl. Physiol.32, 749–754 (1972)
Wranne, B., Woodson, R. D., Detter, J. C.: The two Bohr effects. Physiological consequences of ligand interaction with hemoglobin. In: Hemoglobin and red cell structure and function. Adv. Exp. Med. Biol., vol. 28 (G. Brewer, ed.), pp. 449–455 New-York-London: Plenum Press 1972
Wyman, J.: Linked functions and reciprocal effects in hemoglobin: A second look. Advanc. Protein Chem.19, 223–286 (1964)
Zachara, B.: The effect of inosine, pyruvate and inorganic phosphate on 2,3-diphosphoglycerate, adenine and hypoxanthine nucleotide synthesis in outdated human erythrocytes. J. Lab. clin. Med.85, 436–444 (1975)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duhm, J. Dual effect of 2,3-diphosphoglycerate on the Bohr effects of human blood. Pflugers Arch. 363, 55–60 (1976). https://doi.org/10.1007/BF00587402
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00587402