Abstract
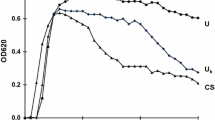
We recently reported that human urine contains a newly identified urinary glycoprotein acting as a potent inhibitor against calcium oxalate crystallization. This inhibitor is a uronic-acid-rich protein (UAP) with a molecular weight of approximately 35 kDa. In the present study, UAP was isolated from urine of stone formers and of subjects without a stone history, and its inhibitory activity was tested in a calcium oxalate crystallization system in vitro. Our results show a weaker inhibitory activity of UAP extracted from the urine of stone formers than that extracted from the urine of healthy subjects. Preliminary analyses of amino acid and carbohydrate content showed some differences between the two groups. The main difference was the reduction in sialic acid in UAP isolated from the urine of stone formers. We suggest that UAP contributes significantly to total urinary inhibitory activity of calcium oxalate crystallization and that the decrease in this activity in the urine of recurrent stone formers is due, in part, to the weak inhibitory activity of UAP. A structural abnormality of UAP could explain the diminution of its inhibitory activity in the urine of stone formers.
Similar content being viewed by others

References
Atmani F, Lacour B, Drüeke T, Daudon M (1993) Isolation and purification of a new glycoprotein from human urine inhibiting calcium oxalate crystallization. Urol Res 21:61
Atmani F, Lacour B, Strecker G, Parvy P, Drüeke T, Daudon M (1993) Molecular characteristics of uronic-acid-rich protein, a strong inhibitor of calcium oxalate crystallization in vitro. Biochem Biophys Res Commun 191:1158
Azoury R, Goldwasser B, Wax Y, Perlberg S, Garti N, Sarig S (1985) Evaluation of the relative inhibitory potential of fractionated urinary macromolecules. Urol Res 13
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330
Bowyer RC, Brockis JG, McCulloch RK (1979) Glycosaminoglycans as inhibitors of calcium oxalate crystal growth and aggregation. Clin Chim Acta 95:23
Caudarella R, Rizzolie E, Martilli G, Berveglieri F, Malavolta N (1988) Urinary excretion of glycosaminoglycans in calcium lithiasis: the role of protein intake. In: Walker VR, Sutton RAL, Cameron ECB, Pak CYC, Robertson WG (eds) Urolithiasis Plenum, Vancouver, p 249
Chambers RE, Clamp JR (1971) An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J 125:1009
Dent CE, Sutor DJ (1971) Presence or absence of inhibitor of calcium-oxalate crystal growth in urine of normals and stoneformers. Lancet II:775
Diferrante N, Rich C (1956) The determination of aminopolysaccharide in urine. J Lab Clin Med 48:491
Foye WO, Hong HS, Kim CM, Prien EL (1976) Degree of sulfation in mucopolysaccharide sulfates in normal and stone-forming urines. Invest Urol 14:33
Hauschka PV (1977) Quantitative determination of γ-carboxyglutamic acid in proteins. Anal Biochem 80:212
Hess B, Nakagawa Y, Coe FL (1989) Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol 257: F99
Hess B, Nakagawa Y, Parks JH, Coe FL (1991) Molecular abnormality of Tamm-Horsfall glycoprotein in calcium oxalate nephrolithiasis. Am J Physiol 260: F569
Itatani H, Itoh H, Yoshioka T, Namiki M, Koide T, Okuyama A, Sonoda T (1981) Renal metabolic changes relating to calculogenesis in an experimental model of calcium containing renal stone formation in rabbits. Invest Urol 19:119
Kitamura T, Zerwekh JE, Pak CYC (1982) Partial biochemical and physicochemical characterization of organic macromolecules in urine from patients with renal stones and control subjects. Kidney Int 21:379
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265
Matsumoto A, Terado T, Kin M, Nishio S, Iwata H, Takeuchi M (1990) Inhibitory factor of calcium oxalate crystal growth in urinary macromolecules. Nippon Hinyokika Gakkai Zasshi 81:883
Morrissey JH (1981) Silver stain for protein in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal Biochem 117:307
Nakagawa Y, Abram V, Kezdy FJ, Kaiser ET, Coe FL (1983) Purification and characterization of the principal inhibitor of calcium oxalate monohydrate crystal growth in human urine. J Biol Chem 258:12594
Nakagawa Y, Abram V, Parks JH, Lau HSH, Kawooya JK, Coe FL (1985) Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. J Clin Invest 76:1455
Renzo C, Mario DR, Vincenzo PC (1979) A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run. Anal Biochem 99:311
Robertson WG, Peacock M (1972) Calcium oxalate crystalluria and inhibitors of crystallization in recurrent renal stone-formers. Clin Sci 43:499
Sallis JD (1987) Glycosaminoglycans as inhibitors of stone formation. Mineral Electrolyte Metab 13:273
Shiraga H, Min W, Vandusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG, Hoyer JR (1992) Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci USA 89:426
Sørensen S, Hansen K, Bak S, Justesen SJ (1990) An unidentified macromolecular inhibitory constituent of calcium oxalate crystal growth in human urine. Urol Res 18:373
Wabner C, Sirivongs D, Maikranz P, Nakagawa Y, Coe FL (1987) Evidence for increased excretion in pregnancy of nephrocalcin (NC), a urinary inhibitor of calcium oxalate (CaOx) crystal growth. Kidney Int 31:359
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atmani, F., Lacour, B., Jungers, P. et al. Reduced inhibitory activity of uronic-acid-rich protein in urine of stone formers. Urol. Res. 22, 257–260 (1994). https://doi.org/10.1007/BF00541903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00541903