Abstract
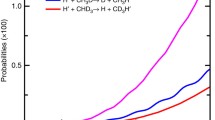
According to experimental data, gas-phase SN2 reactions have low efficiency characterized by the ratio k(T)/kc, where kc is the collision rate constant. The energy profile of the reaction pathway is a double-well curve, and the top of the central barrier may be located either above or below the energy level of the reagents. Nonempirical calculations of the potential surfaces for SN2 reactions have shown that their dynamics may be described in the collinear collision approximation. The probability of a reactive collision is determined by the interference of direct processes and processes of decay of quasibound states arising upon formation of the pre-reaction complex. The reflection probability in the direct process is determined by the degree of excitation of the reactive system in channels that are closed at the central barrier point, which depends on the curvature of the reaction pathway and the depth of the potential well for the pre-reaction complex. Depending on these factors, the direct process may correspond either to total transmission or to total reflection (low reaction efficiency). In the first case, interference of direct processes with decay processes decreases the reaction probability; in the second case, such interference increases the reaction probability. Calculations of the thermal reaction rate constants k(T) have shown good agreement with experimental data.
Similar content being viewed by others
Literature cited
K. Tanaka, G. I. Mackay, J. D. Payzant, and D. K. Bohme, “Gas-phase reactions of anions with halogenated methanes at 297+2°K,” Can. J. Chem., 54, No. 10, 1643–1659 (1976).
W. N. Olmstead and J. I. Brauman, “Gas-phase nucleophilic displacement reactions,” J. Am. Chem. Soc., 99, No. 13, 4219–4228 (1977).
M. J. Pellerite and J. I. Brauman, “Intrinsic barriers in nucleophilic displacements,” J. Am. Chem. Soc., 102, No. 19, 5993–5939 (1980).
T. Su and M. T. Bowers, “Theory of ion-polar molecule collisions. Comparison with experimental charge-transfer reactions of rare gas ions to geometric isomers of difluorobenzene and dichloroethylene,” J. Chem. Phys., 58, No. 7, 3027–3037 (1973).
P. J. Robinson and K. A. Holbrook, Unimolecular Reactions [Russian translation], Mir, Moscow (1975).
V. M. Ryaboi, “Nonempirical calculations of potential surfaces for gas-phase nucleophilic substitution reactions (SN2),” Teor. Eksp. Khim., 22, No. 3, 271–281 (1986).
M. V. Basilevsky and V. M. Ryaboy, “Quantum investigation of linear triatomic exchange reactions. A computational method,” Chem. Phys., 41, No. 3, 461–476 (1979).
J. C. Light and R. B. Walker, “An R-matrix approach to the solution of coupled equations for atom-molecule reactive scattering,” J. Chem. Phys., 65, No. 10, 4272–4282 (1976),
S. Wolfe, D. J. Mitchell, and H. B. Schlegel, “Theoretical studies of SN2 transition states,” J. Am. Chem. Soc., 103, No. 24, 7692–7694 (1981).
M. Urban, I. Černušak, and V. Kellö, “Activation barriers of SN2 reactions F− + CH3F and H− + CH3F. Four-order MB RSPT calculations,” Chem. Phys. Lett., 105, No. 6, 625–629 (1984).
M. V. Basilevsky and V. M. Ryaboy, “Dynamics of linear exchange reactions. Quasiclassical model of high-energy vibrational inversion,” Chem. Phys., 41, No. 3, 477–488 (1979).
J. M. Bowman, G.-Zh. Ju, and K. T. Lee, “Incorporation of collinear exact quantum probabilities into three-dimensional transition state theory,” J. Phys. Chem., 86, No. 12, 2232–2239 (1982).
G. Caldwell, J. F. Magnera, and P. Kerable, “SN2 reactions in the gas phase. Temperature dependence of the rate constants and energies of the transition states. Comparison with solution,” J. Am. Chem. Soc., 106, No. 4, 959–966 (1984).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 22, No. 4, pp. 435–443, July–August, 1986.
The author acknowledges M. V. Bazilevskii, A. M. Berezhkovskii, V. A. Tikhomirov, and G. É. Chudinov for useful discussions.
Rights and permissions
About this article
Cite this article
Ryaboi, V.M. Quantum dynamic calculation for gas-phase nucleophilic substitution (SN2) reactions. Theor Exp Chem 22, 417–424 (1987). https://doi.org/10.1007/BF00523819
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00523819