Abstract
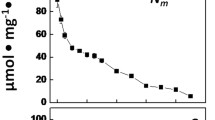
Simultaneous measurements of acetylene reduction by Anabaena variabilis and the concentration of dissolved oxygen in the suspension were made using a specially designed vessel which allowed measurements under steady-state conditions. The rate of acetylene reduction in the dark increased with increasing oxygen concentrations until a maximum value was reached at 300 μM O2 (corresponding to 30% O2 in the gas phase at 35°C). This presumably results from a requirement for energy provided by respiration. Measurements of the dependence of respiration rate on dissolved oxygen concentration were made under comparable conditions using an open system to allow conditions close to steady-state to be obtained. The respiration rate of diazotrophically grown Anabaena variabilis had a dependence on oxygen concentration corresponding to the sum of two activities. These had K m values of 1.0 μM and 69 μM and values of V max of similar magnitude. Only the high affinity activity was observed in nitrate-grown cyanobacteria lacking heterocysts, and this presumably represent activity in the vegetative cells. The oxygen concentration dependence of the low affinity activity resembled that for the stimulation of acetylene reduction. We interpret this as the result of oxygen uptake by the heterocysts. The results are consistent with the idea that in intact filaments of cyanobacteria O2 enters heterocysts much more slowly than it enters the vegetative cells.
Similar content being viewed by others
References
Bergersen FJ, Turner GL (1980) Properties of terminal oxidase systems of bacteroids from root nodules of soybean and cowpea and of N2 fixing bacteria grown in continuous culture. J Gen Microbiol 118:235–252
Booker MJ, Walsby AE (1979) The relative form resistance of straight and helical blue-green algal filaments. Br Phycol J 14:141–150
Burns DJW, Tucker SA (1977) An evaluation of fitting methods for the sum of two hyperbolas: application to uptake studies. Eur J Biochem 81:45–52
Chemical Rubber Company (1962) Handbook of chemistry andphysics. 44th ed. CRC Press, Cleveland, OH, pp 1706 and 1708
Degn H, Lundsgaard JS, Petersen LC, Ormicki A (1980) Polarographic measurement of steady-state kinetics of oxygen uptake by biochemical samples. Meth Biochem Anal 26:47–77
Degn H, Wohlrab H (1971) Measurement of steady-state values of respiration rate and oxidation levels of respiratory pigments at low oxygen tensions. A new technique. Biochim Biophys Acta 245:347–355
Eisenthal R, Cornish-Bowden A (1974) The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J 139:715–720
Falkenberg P, Buckland B, Walsby AE (1972) Chemical composition of gas vesicles isolated from Anabaena flos-aquae. Arch Mikrobiol 85:304–309
Fay P (1980) Heterocyst isolation. Methods Enzymol 69:801–812
Fay P, Walsby AE (1966) Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature (Lond) 209:94–95
Gallon JR (1981) The oxygen sensitivity of nitrogenase: a problem for biochemists and micro-organisms. Trends Biochem Sci 6:19–23
Haury JF, Wolk CP (1978) Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J Bact 136:688–692
Haystead A, Robinson R, Stewart WDP (1970) Nitrogenase activity in extracts of heterocystous and non-heterocystous blue-green algae. Arch Mikrobiol 74:235–243
Hochman A, Burris RH (1981) Effect of oxygen on acetylene reduction by photosynthetic bacteria. J Bact 147:492–499
Jensen BB, Cox RP (1983) Direct measurements of steady-state kinetics of cyanobacterial N2 uptake using membrane-leak mass spectrometry, and comparisons between nitrogen fixation and acetylene reduction. Appl Environ Microbiol 45:1331–1337
Linton JD, Bull AT, Harrison DEF (1977) Determination of the apparent K m for oxygen of Beneckea natriegens using the respirograph technique. Arch Microbiol 114:111–113
Lloyd D, Williams J, Yarlett N, Williams AG (1982) Oxygen affinities of the hydrogenosome-containing protozoa Tritrichomonas foetus and Dasytericha ruminantium, and two aerobic protozoa, determined by bacterial bioluminescence. J Gen Microbiol 128:1019–1022
Lundsgaard JS, Degn H (1973) Digital regulation of gas flow rates and composition of gas mixtures. IEEE Trans Biomed Eng 20:384–387
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Peschek GA, Schmetterer G, Lauritsch G, Nitschmann WH, Kienzl PF, Muchl R (1982) Do cyanobacteria contain “mammalian-type” cytochrome oxidase? Arch Microbiol 131:261–265
Petersen LC (1977) The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta 460:299–307
Petersen LC, Nicholls P, Degn H (1976) The effect of oxygen concentration on the steady-state kinetics of the solubilized cytochrome c oxidase. Biochim Biophys Acta 452:59–65
Peterson RB, Burris RH (1976) Properties of heterocysts isolated with colloidal silica. Arch Microbiol 108:35–40
Peterson RB, Burris RH (1978) Hydrogen metabolism in isolated heterocysts of Anabaena 7120. Arch Microbiol 116:125–132
Rice CW, Hempfling WP (1978) Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bact 134:115–124
Robson RL, Postgate JR (1980) Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol 34:183–207
Stewart WDP (1973) Nitrogen fixation by photosynthetic microorganisms. Annu Rev Microbiol 27:283–316
Tel-Or E, Stewart WDP (1977) Photosynthetic components and activities of nitrogen fixing isolated heterocysts of Anabaena cylindrica. Proc R Soc Lond B 198:61–86
Tischer RG (1965) Pure culture of Anabaena flos-aquae A 37. Nature (Lond) 205:419–420
Walsby AE (1982) The permeability of gas vesicles, vegetative cells and heterocysts of Anabaena flos-aquae to oxygen and nitrogen. Abstracts IV International Symposium on Photosynthetic Prokaryotes, Bombannes, France, C33
Watanabe A, Yamamoto Y (1967) Heterotrophic nitorgen fixation by the blue-green alga Anabaenopsis circularis. Nature (Lond) 214:738
Wolk CP (1982) Heterocysts. In: Carr NG, Whitton BW (eds) The biology of cyanobacteria. Blackwell, Oxford, pp 359–386
Wolk CP, Shaffer PW (1976) Heterotrophic micro- and macro-cultures of a nitrogen-fixing cyanobacterium. Arch Microbiol 110:145–147
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, B.B., Cox, R.P. Effect of oxygen concentration on dark nitrogen fixation and respiration in cyanobacteria. Arch. Microbiol. 135, 287–292 (1983). https://doi.org/10.1007/BF00413483
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00413483