Abstract
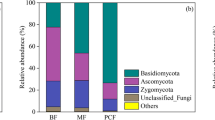
In a field study using soil mesocosms in an acid spruce forest soil we investigated the effects of mesofauna and macrofauna on microbial biomass, dissolved organic matter, and N cycling. Intact soil monoliths were taken from the ground, defaunated by deep-freezing, and wrapped in nets of various mesh-sizes to control re-immigration of different faunal size-classes. The monoliths were then replanted in the field. Three treatments of mesocosms were prepared: (1) with only microbiota, (2) microbiota and mesofauna, and (3) microbiota, mesofauna, and macrofauna (= complex fauna). After 8 months of exposure the mesocosms and the unmanipulated control plots (treatment 4) were destructively sampled. We estimated microbial biomass by substrate-induced respiration and the chloroform fumigation-extraction method. N cycling was measured by monitoring microbial N mineralization, the NH sup+inf4 content, and selected amino acids and the activities of protease, urease, and deaminase. The results from the L/F layer showed that the pool of the microbial biomass was not changed by the activity of the mesofauna. However, the mesofauna and macrofauna together enhanced SIR. An increase in microbial N mineralization was only observed in treatment 3 (microbiota + complex fauna). Protease activity and NH sup+inf4 content increased in treatments 2 (microbiota + mesofauna) and 3 (microbiota + complex fauna). The complex fauna induced a soil pH increase in treatment 3 as opposed to treatment 1 and the control. This increase was presumably due to excretory NH sup+inf4 . Principal component analysis revealed that the complex fauna in treatment 3 caused a significantly higher N turnover per unit of microbial biomass.
Similar content being viewed by others
References
Abrahamsen G (1990) Influence of Cognettia sphagnetorum (Oligochaeta: Enchytraeidae) on nitrogen mineralization in homogenized mor humus. Biol Fertil Soils 9:159–162
Alef K, Kleiner D (1986) Arginine ammonification, a simple method to estimate microbial activity potentials in soils. Soil Biol Biochem 18:233–235
Amato M, Ladd JN (1988) Assay for microbial biomass based on ninhydrin-reactive nitrogen in extracts of fumigated soils. Soil Biol Biochem 10:107–114
Anderson JM (1987) Interactions between invertebrates and microorganisms: Noise or necessity for soil processes? In: Fletcher M, Gray TRG (eds) Ecology of microbial communities. Cambridge University Press, New York, pp 125–145
Anderson JPE, Domsch KH (1978) A physiological method for quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (q CO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Anderson JM, Ineson P (1983) The effects of animal feeding activities on element release from deciduous forest litter and soil organic matter. In: Lebrun P, Andre HM, de Medts A, Gregoire-Wibo C, Wauthy G (eds) Proc VIII Int Colloq Soil Zool Dieu-Brichard, Ottignies-Louvian-la-Neuve, pp 87–100
Anderson JM, Ineson P, Huish SA (1983) Nitrogen and cation mobilization by soil fauna feeding on leaf litter and soil organic matter from deciduous woodland. Soil Biol Biochem 15:463–467
Anderson JM, Huish SA, Ineson P, Leonard MA, Splatt PR (1985) Interactions of invertebrates, micro-organisms and tree roots in nitrogen and mineral element fluxes in deciduous woodland soils. In: Fitter AH, Atkinson D, Read DJ, Usher MB (eds) Ecological interactions in soil: Plants, microbes and animals. Blackwell Scientific Publications, Palo Alto, pp 377–392
Bachmann G, Kinzel H (1992) Physiological and ecological aspects of the interaction between plant roots and rhizosphere soil. Soil Biol Biochem 24:543–552
Barber DA, Gunn KB (1974) The effect of mechanical forces on exudation of organic substances by the roots of cereal plants grown under sterile conditions. New Phytol 73:39–45
Bauer R, Kampichler C, Kandeler E, Bruckner A (1994) Enchytraeids (Oligochaeta) in an Austrian spruce forest: Abundance, biomass, vertical distribution and re-immigration into defaunated mesocosms. Eur J Soil Biol 30:143–148
Beare MH, Parmelee RM, Hendrix PF, Cheng W, Coleman DC, Crossley Jr DA (1992) Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecological Monograph 62:569–591
Beck L (1987) Lebensraum Buchenwald 1. Bodenfauna und Streuabbau — eine Übersicht. Verh Ges Ökol (Göttingen) 17:47–51
Borkott H (1989) Elementgehalte (C, N, P, K) wirbelloser Bodentiere. Z Pflanzenernaehr Bodenkd 152:77–80
Bruckner A, Kampichler C, Wright J, Bauer R, Kandeler E (1993) Using mesocosms to investigate mesofaunal-microbial interactions in soil: Re-immigration of fauna to defaunated monoliths. Mitt Dtsch Bodenkd Ges 69:151–154
Bruckner A, Wright J, Kampichler C, Kandeler E (1995) A method to prepare mesocosms for assessing complex biotic processes in soils. Biol Fertil Soils 19:257–262
Coleman DC (1986) The role of microfloral and faunal interactions in affecting soil processes. In: Mitchell MJ, Nakas JP (eds) Microfloral and faunal interactions in natural and agro-ecosystems. Martinus Nijhoff/W. Junk, Dordrech, pp 317–348
Cooke RC, Rayner ADM (1984) Ecology of saprotrophic fungi. Longman, New York
Faber JH, Verhoef HA (1991) Functional differences between closely related soil arthropods with respect to decomposition processes in the presence or absence of pine tree roots. Soil Biol Biochem 23:15–23
Dunger W, Fiedler HJ (1989) Methoden der Bodenbiologie. Gustav Fischer Verlag, Stuttgart
Flury B, Riedwyl H (1983) Angewandte multivariate Statistik. Gustav Fischer Verlag, Stuttgart
Frankland JC (1981) Mechanisms in fungal successions. In: Wicklow DT, Carroll GC (eds) The fungal community. Dekker, New York, pp 403–426
Hanlon RDG (1981) Influence of grazing by collembola on the activity of senescent fungal colonies on media of different nutrient concentration. Oikos 36:362–367
Hanlon RDG, Anderson JM (1979) The effects of collembola grazing on microbial activity in decomposing leaf litter. Oecologia 38:93–99
Hedlund K, Boddy L, Preston CM (1991) Mycelial responses of the fungus, Mortierella isabellina, to grazing by Onychiurus armatus (Collembola). Soil Biol Biochem 23:361–366
Ineson P, Leonard MA, Anderson JM (1982) Effect of collembolan grazing upon nitrogen and cation leaching from decomposing leaf litter. Soil Biol Biochem 14:601–605
Ingelög T, Nohrstedt H-Ö (1993) Ammonia formation and soil pH increase caused by decomposing fruitbodies of macrofungi. Oecologia 93:449–451
Ingham RE, Trofymow JA, Ingham ER (1985) Interactions of bacteria, fungi and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140
Jäggi W (1976) Die Bestimmung der CO2-Bildung als Mass der bodenbiologischen Aktivität. Schweiz Landwirtsch Forsch 15:371–380
Jongman RH, ter Braak CJF, van Tongeren OFR (1987) Data analyses in community and landscape ecology. Centre for Agricultural Publishing and Documentation (Pudoc). Wageningen
Kampichler C (1995) Biomass distribution of a microarthropod community in spruce forest soil. Biol Fertil Soils 19:263–265
Kampichler C, Bruckner A, Kandeler E, Bauer R, Wright J (1995) A mesocosm study design using undisturbed soil monoliths. Acta Zool Fenn 196:71–72
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kandeler E, Winter B, Kampichler C, Bruckner A (1994) Effects of mesofaunal exclusion on microbial biomass and patterns on enzymatic activities in field mesocosms. In: Ritz K, Dighton J, Giller K (eds) Beyond the biomass. Wiley and Sons, Chichester, pp 181–189
Keeney DC (1982) Nitrogen-availability indices. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Am Soc Agron Madison, Wis, pp 711–733
Ladd JN, Butler JHA (1972) Short-term assay of soil proteolytic enzyme activities using proteins and dipeptide derivates as substrates. Soil Biol Biochem 4:19–30
Leonard MA, Anderson JM (1991) Growth dynamics of collembola (Folsomia candida) and a fungus (Mucor plumbeus) in relation to nitrogen availability in spatial simple and complex laboratory systems. Pedobiologia 35:163–173
Linher O (1990) Die Bedeutung des Gehaltes freier niedermolekularer Substanzen im Boden unter besonderer Berücksichtigung der Wechselwirkung Wurzel-Rhizosphäre-Boden. PhD thesis, Univ Vienna
Lussenhop J (1992) Mechanisms of microarthropod-microbial interactions in soil. Adv Ecol Res 23:1–33
Martens R (1982) Apparatus to study the quantitative relationships between root exudates and microbial populations in the rhizosphere. Soil Biol Biochem 14:315–317
Newell K (1984) Interaction between two decomposer basidiomycetes and a collembolan under Sitka spruce: Grazing and its potential effects on fungal distribution and litter decomposition. Soil Biol Biochem 16:235–239
Odum EP (1984) The mesocosm. BioScience 34:558–562
Parkinson D, Visser S, Whittaker JB (1979) Effect of collembolan grazing on fungal colonization of leaf litter. Soil Biol Biochem 11:529–535
Paul EA, Clark FE (1989) Soil as habitat for organisms and their reactions. In: Paul EA, Clark FE (eds) Soil microbiology and biochemistry. Academic Press, London, pp 11–30
Scholle G, Wolters U, Jörgensen RG (1992) Effects of mesofauna exclusion on the microbial biomass in two moder profiles. Biol Fertil Soils 12:253–260
Seastedt TR (1984) The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol 29:25–46
Setälä H, Huhta V (1991) Soil fauna increase Betula pendula growth: Laboratory experiments with coniferous forest floor. Ecology 72:665–671
Setälä H, Martikainen E, Tyynismaa M, Huhta V (1990) Effects of soil fauna on leaching of nitrogen and phosphorus from experimental systems simulating coniferous forest floor. Biol Fertil Soils 10:170–177
Setälä H, Tyynismaa M, Martikainen E, Huhta V (1991) Mineralization of C, N and P in relation to decomposer community structure in a coniferous forest soil. Pedobiologia 35:285–296
Shaw Z, Adams WA, Haven CDV (1990) Composition and activity of the microbial population in an acid upland soil and effects of liming. Soil Biol Biochem 22:257–263
Sparling GP (1985) The soil biomass. In: Vaughan D, Malcolm RE (eds) Soil organic matter and biological activity. Nijhoff, Dordrecht, pp 223–262
Tate K, Ross DJ, Feltham CW (1988) A direct extraction method to estimate soil microbial C: Effects of experimental variables and some different calibration procedures. Soil Biol Biochem 20:329–335
Teuben A (1991) Nutrient availability and interactions between soil arthropods and microorganisms during decomposition of coniferous litter: A mesocosm study. Biol Fertil Soils 10:256–266
Teuben A, Roelofsma TAPJ (1990) Dynamic interactions between functional groups of soil arthropods and microorganisms during decomposition of coniferous litter in microcosm experiments. Biol Fertil Soils 9:145–151
Verhoef HA, Brussaard L (1990) Decomposition and nitrogen mineralization in natural and agroecosystems: The contribution of soil animals. Biogeochemistry 11:175–211
Verhoef HA, de Goede RGM (1985) Effects of collembolan grazing on nitrogen dynamics in a coniferous forest soil. In: Fitter AH, Atkinson D, Read DJ, Usher MB (eds) Ecological interactions in soil: plants, microbes and animals. Blackwell Scientific Publications, Palo Alto, pp 367–376
Visser S (1985) Role of soil invertebrates in determining the composition of soil microbial communities. In: Fitter AH, Atkinson D, Read DJ, Usher MB (eds) Ecological interactions in soil: Plants, microbes and animals. Blackwell Scientific Publications, Palo Alto, pp 297–317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vedder, B., Kampichler, C., Bachmann, G. et al. Impact of faunal complexity on microbial biomass and N turnover in field mesocosms from a spruce forest soil. Biol Fert Soils 22, 22–30 (1996). https://doi.org/10.1007/BF00384428
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00384428