Summary
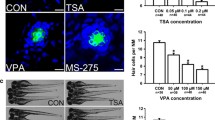
External application of 10 rig/ml (R)-trichostatin A (TSA), a potent and specific inhibitor of mammalian histone deacetylase, to the embryo of the starfish Asterina pectinifera inhibited development during the early gastrula stage before formation of mesenchyme cells. The TSA-sensitive period was limited to the mid-blastula stage before hatching. The pulse-chase experiment clearly demonstrated that TSA induced an accumulation of acetylated histone species in blastulae through inhibition of historic deacetylation. Similar blockage of development at the early gastrula stage was observed with n-butyrate, which has been known as a weak inhibitor of historic deacetylase. These results suggest an intimate role for historic acetylation-deacetylation equilibria in starfish development.
Similar content being viewed by others
References
Allegra P, Sterner R, Clayton DF, Allfrey VG (1987) Affinity chromatographic purification of nucleosomes containing transcriptionally active DNA sequences. J Mol Biol 196:379–388
Cousens LS, Gallwitz D, Alberts BM (1979) Differential accessibilities in chromatin to histone acetylase. J Biol Chem 254:1716–1723
Csordas A (1990) On the biological role of histone acetylation. Biochem J 265:23–38
D'Anna JA, Tobey RA, Gurley LR (1980) Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cellcycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry 19:2656–2671
Gorman CM, Howard BH, Reeves R (1983) Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res 11:7631–7648
Hebbes TR, Thorne AW, Crane-Robinson C (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 7:1395–1403
Ikegami S (1991) Chromatin maturation during early development of the starfish, Asterina pectinifera. In: Hoshi M, Yamashita O (eds) Advances in invertebrate reproduction, vol 5. Elsevier, Amsterdam, pp 75–80
Ikegami S, Ozaki Y, Ooe Y, Itch N (1991) Achromosomal division of early starfish embryos cultured in the presence of actinomycin D. Growth Dev Differ 33:193–200
Isomura H, Itch N, Ikegami S (1989) RNA synthesis in starfish embryos: developmental consequences of its inhibition by formycin. Biochim Biophys Acta 1007:343–349
Johnson EM, Sterner R, Allfrey VG (1987) Altered nucleosomes of active nucleolar chromatin acessible histone H3 in its hyperacetylated forms. J Biol Chem 262:6943–6946
Klehr D, Schlake T, Maass K, Bode J (1992) Scaffold-attached regions (SAR elements) mediate transcriptional effects due to butyrate. Biochemistry 31:3222–3229
Kominami T, Satoh N (1980) Temporal and cell-numerical organization of embryos in the starfish, Asterina pectinifera. Zool Mag (Tokyo) 89:244–251
Morioka H, Ishihara M, Takezawa M, Shibai H, Komoda Y (1988) (+)-Trichostatin A, a potent differentiation inducer of Friend leukemia cells. Agric Biol Chem 52:251–253
Nagano H, Hirai S, Okano K, Ikegami S (1981) Achromosomal cleavage of fertilized starfish eggs in the presence of aphidicolin. Dev Biol 85:409–415
Ridsdale JA, Davie JR (1987) Chicken erythrocyte polynucleosomes which are soluble at physiological ionic strength and contain linker histones are highly enriched in β-globin gene sequences. Nucleic Acids Res 15:1081–1096
Riggs MG, Whittaker RG, Neumann JR, Ingram VM (1977) n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 268:462–464
Sealey L, Chalkley R (1978) The effect of sodium butyrate on histone modification. Cell 14:115–121
Tsuchimori N, Miyashiro S, Shibai H, Ikegami S (1988) Adenosine induces dormancy in starfish blastulae. Development 103:343–351
Tsuji N, Kobayashi M, Nagashima K, Wakisaka Y, Koizumi KA (1976) A new antifungal antibiotic, trichostachin. J Antibiot 29:1–6
Turner BM (1991) Historic acetylation and control of gene expression. J Cell Sci 99:13–20
Yamada H, Hirai S, Ikegami S, Kawarada Y, Okuhara E, Nagano H (1985) The fate of DNA originally existing in the zygote nucleus during achromosomal cleavage of fertilized echinoderm eggs in the presence of aphidicolin: microscopic studies with anti-DNA antibody. J Cell Physiol 124:9–12
Yoshida M, Beppu T (1988) Reversible arrest of proliferation of rat 3Y1 fibroblasts in both the G1 and G2 phases by trichostatin A. Exp Cell Res 177:122–131
Yoshida M, Iwamoto Y, Uozumi T, Beppu T (1985) Trichostatin C, a new inducer of differentiation of Friend leukemic cells. Agric Biol Chem 49:563–565
Yoshida M, Nomura S, Beppu T (1987) Effects of trichostatins on differentiation of murine erythroleukemia cells. Cancer Res 47:3688–3691
Yoshida M, Kijima M, Akita M, Beppu T (1990) Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by Trchostatin A. J Biol Chem 265:17174–17179
Author information
Authors and Affiliations
Additional information
Correspondence to: S. Ikegami
Rights and permissions
About this article
Cite this article
Ikegami, S., Ooe, Y., Shimizu, T. et al. Accumulation of multiacetylated forms of histones by trichostatin A and its developmental consequences in early starfish embryos. Roux's Arch Dev Biol 202, 144–151 (1993). https://doi.org/10.1007/BF00365304
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00365304