Abstract
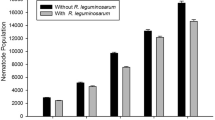
The effects of inoculating field peas (Pisum sativum L.) with Rhizobium leguminosarum and field beans (Phaseolus vulgaris L.) with R. phaseoli, alone or in combination with Pseudomonas syringae R25 and/or P. putida R105, were assessed under gnotobiotic conditions in growth pouches and in potted soil in a growth chamber. Inoculation of peas with P. syringae R25 or P. putida R105 alone had no effect on plant growth in pouches. In soil, however, the isolate R25 inhibited nitrogenase activity (as assessed by acetylene reduction assay) of nodules formed by indigenous rhizobia; strain R105 stimulated pea seedling emergence and nodulation. P. syringae R25 inhibited the growth of beans in either plant-growth system. P. putida R105, however, had no effects on beans in pouches, but reduced plant root biomass and nodulation by indigenous rhizobia in soil. Coinoculation of pea seeds with R. leguminosarum and either of the pseudomonads significantly (P<0.01) increased shoot, root, and total plant weight in growth pouches, but had no effect in soil. Co-inoculation of field beans with R. phaseoli and P. putida R105 had no effects on plant biomass in growth pouches or in soil, but the number of nodules and the acetylene reduction activity was significantly (P<0.01) increased in the soil. In contrast, co-inoculation of beans with rhizobia and P. syringae R25 had severe, deleterious effects on seedling mergence, plant biomass, and nodulation in soil and growth pouches. Isolate R25 was responsible for the deleterious effects observed. Although plant growth-promoting rhizobacteria may interact synergistically with root-nodulating rhizobia, the PGPR selected for one crop should be assessed for potential hazardous effects on other crops before being used as inoculants.
Similar content being viewed by others
References
Al-Mallah MK, Davey MR, Cocking DE (1987) Enzymatic treatment of clover root hairs removes a barrier to rhizobium—host specificity. Biotechnology 5:1319–1322
Alström S (1987) Factors associated with detrimental effects of rhizobacteria on plant growth. Plant and Soil 102:3–9
Azcón AC, Barea JM (1978) Effects of interactions between different culture fractions of “phosphobacteria” and Rhizobium on mycorrhyzal infection, growth and nodulation of Medicago sativa. Can J Microbiol 24:520–524
Azcón R, Azcón AC, Barea JM (1978) Effects of plant hormones present in bacterial cultures on the formation and responses to VA mycorrhiza. New Phytol 80:359–364
Beringer JE (1974) R-factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Burr TJ, Schroth MN, Suslow T (1978) Increased potato yields by treatment of seed pieces with specific strains of Pseudomonas fluorescens and Pseudomonas putida. Phytopathology 68:1377–1383
Chanway CP, Hynes RK, Nelson LM (1989) Plant growth-promoting rhizobacteria: effects on growth and nitrogen fixation of lentil (Lens esculenta Moench) and pea (Pisum sativum L.). Soil Biol Biochem 21:511–517
De Freitas JR, Germida JJ (1990) Plant growth promoting rhizobacteria for winter wheat. Can J Microbiol 36:265–272
Fuhrmann J, Wollum AG II (1989) In vitro growth responses of Bradyrhizobium japonicum to soybean rhizosphere bacteria. Soil Biol Biochem 21:131–135
Germida JJ (1988) Growth of indigenous Rhizobium leguminosarum and Rhizobium meliloti in soils amended with organic nutrients. Appl Environ Microbiol 54:257–263
Grimes HD, Mount MS (1984) Influence of Pseudomonas putida on nodulation of Phaseolus vulgaris. Soil Biol Biochem 16:27–30
Hicks PM, Loynachan TW (1989) Bacteria of the soybean rhizosphere and their effect on growth of Bradyrhizobium japonicum. Soil Biol Biochem 24:561–566
Hoagland DR, Arnon DI (1938) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn No 347
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Kloepper JW, Schroth MN, Miller TD (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70:1078–1382
Knight TJ, Langston-Unkefer PJ (1988) Enhancement of symbiotic dinitrogen fixation by a toxin-releasing plant pathogen. Science 241:951–954
Okon Y, Hadar Y (1987) Microbial inoculants as crop-yield enhancers. CRC Crit Rev Biotechnol 6:61–85
Polonenko DR, Scher MF, Kloepper JW, Singleton CA, Laliberte M, Zaleska I (1987) Effects of root colonizing bacteria on nodulation of soybean roots by Bradyrhizobium japonicum. Can J Microbiol 33:498–503
Rai R (1983) Efficacy of associative N2-fixation by streptomycin-resistant mutants of Azospirillum brasilense with genotypes of chick pea Rhizobium strains. J Agric Sci 100:75–80
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill Inc, Toronto
Suslow TV (1982) Role of root-colonizing bacteria in plant growth. In: Mount MS, Lacey G (eds) Phytopathogenic prokaryotes vol 1 Academic Press, New York, pp 187–223
Vincent JM (1970) A manual for the practical study of root nodule bacteria. International Biological Programme Handbook 15, Blackwell, Oxford
Yahalom E, Okon Y, Dovrat A (1987) Azospirillum effects on susceptibility to Rhizobium nodulation and on nitrogen fixation of several forage legumes. Can J Microbiol 33:510–514
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Freitas, J.R., Gupta, V.V.S.R. & Germida, J.J. Influence of Pseudomonas syringae R25 and P. putida R105 on the growth and N2 fixation (acetylene reduction activity) of pea (Pisum sativum L.) and field bean (Phaseolus vulgaris L.). Biol Fertil Soils 16, 215–220 (1993). https://doi.org/10.1007/BF00361411
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00361411