Summary
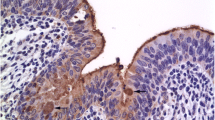
To elucidate the putative role of annexin II (calpactin I) in the secretory function of mammary tissue its immunolocalisation in the mammary gland of pregnant and lactating mice was investigated by light- and electron microscopy using the immunoperoxidase technique. A low level of fairly uniform annexin II staining was evident throughout the gland despite its mixed composition during pregnancy. In lactating tissue it was revealed that apparently mature alveoli contained a concentration of annexin II staining outlining their epithelium. The staining was localised by immuno-electron microscopy to the apical membrane of these alveolar epithelial cells and their microvillar extentions. There was also an apparent association of annexin II with vesicles of a range of sizes located near, or actually fused with, the apical membrane. Many of the small, stained vasicles could clearly be identified as casein-containing vesicle while the large vesicles were apparently associated with either casein granules or possibly lipid. The appearance of a selective concentration of annexin II in apparently actively secreting mammary epithelial cells, as revealed in this study, is consistent with a possible structural and/or functional role for this protein at the membranes participating in the secretion of protein and possibly lipid from these secretory cells.
Similar content being viewed by others
References
Ali SM, Burgoyne RD (1990) The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signall 2:265–276
Ali SM, Geisow MJ, Burgoyne RD (1989) A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature 340:313–315
Blackwood RA, Ernst JD (1990) Characterization of Ca2+-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J 266:195–200
Braslau DL, Ringo DL, Rocha V (1984) Synthesis of novel calcium-dependent proteins associated with mammary epithelial cells migration and differentiation. Exp Cell Res 155:213–221
Burgoyne RD, Cheek TR (1987) Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci Rep 7:281–288
Burgoyne RD, Geisow MJ (1989) The annexin family of calcium-binding proteins. Cell Calcium 10:1–10
Burgoyne RD, Morgan A (1990) Evidence for a role of calpactin in calcium-dependent exocytosis. Biochem Soc Trans 18:1111–1114
Burgoyne RD, Cheek TR, Morgan A, O'Sullivan AJ, Moreton RB, Berridge MJ, Mata AM, Colyer J, Lee AG, East JM (1989) Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature 342:72–74
Cheng YSE, Chen LB (1981) Detection of phosphotyrosine-containing 36000 dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci USA 78:2388–2392
Drust DS, Creutz CE (1988) Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature 331:88–91
Franke WW, Luder MR, Kartenbeck J, Zerban H, Keenan TW (1976) Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol 69:173–195
Geisow MJ, Walker JH, Boustead C, Taylor W (1987) Annexins-new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep 7:289–298
Gerke V (1989) Tyrosine protein kinase substrate p36: a member of the annexin family of Ca2+/phospholipid binding proteins. Cell Motil Cytoskel 14:449–454
Gerke V, Weber K (1984) Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J 3:227–233
Gerke V, Weber K (1985) Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem 260:1688–1695
Glenney JR, Tack B, Powell MA (1987) Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol 104:503–511
Hom YK, Sudhof TC, Lozano JJ, Haindl AH, Rocha V (1988) Mammary gland Ca++-binding (-dependent) proteins: identification as calelectrins and calpactin I/p 36. J Cell Physiol 135:435–442
Jamieson AM, Paschke M, Clegg RA (1990) Mammary tissue lipocortins of the lactating rat. Biochem Soc Trans 18:1126–1127
Klee CB (1988) Ca++-dependent phospholipid- (and membrane-) binding proteins. Biochemistry 27:6645–6653
Lehto V-P, Virtanen I, Paasivuo R, Ralston R, Alitalo K (1983) The p36 substrate of tyrosine-specific protein kinases co-localizes with non-erythrocyte-spectrin antigen, p230, in surface lamina of cultured fibroblasts. EMBO J 2:1701–1705
Lozano JJ, Silberstein GB, Hwang S-I, Haindl AH, Rocha V (1989) Developmental regulation of calcium-binding proteins (calelectrins and calpactin I) in mammary glands. J Cell Physiol 138:503–510
Nakata T, Sobue K, Hirokawa N (1990) Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy. J Cell Biol 110:13–25
Semich R, Gerke V, Robenek H, Weber K (1989) The p36 substrate of pp60src kinase is located at the cytoplasmic surface of the plasma membrane of fibroblasts; an immunoelectron microscopic analysis. Eur J Cell Biol 50:313–323
Schlaepfer DD, Haigler HT (1990) Expression of annexins as a function of cellular growth state. J Cell Biol 111:229–238
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Handel, S.E., Rennison, M.E., Wilde, C.J. et al. Annexin II (calpactin I) in the mouse mammary gland: immunolocalisation by light- and electron microscopy. Cell Tissue Res 264, 549–554 (1991). https://doi.org/10.1007/BF00319044
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319044