Summary
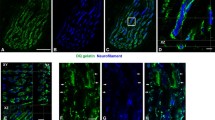
The immunocytochemical expression of two myosin isoforms in intrafusal muscle fibers was examined in soleus muscles of neonatal (zero to six days postpartum) and adult rats. Monoclonal antibodies specific for myosin heavy chains of the slow-tonic anterior latissimus dorsi (ALD58) and fast-twitch pectoralis (MF30) muscles of the chicken were used. In adults ALD58 bound to the intracapsular regions of bag1 and bag2 fibers and MF30 bound to the intracapsular regions of bag2 and chain fibers. The extracapsular regions of intrafusal fibers and all extrafusal fibers did not react to ALD58 or MF30. Bag1 and bag2 fibers of neonatal rats expressed immature myosin patterns but chain fibers did not. The adult pattern of immunoreactivity of intrafusal fibers developed by the fourth postnatal day, when the patterns of motor but not sensory innervation in the spindle are still immature. Data suggest that the expression and maintenance of the specific anti-myosin immunoreactivity of intrafusal fibers during postnatal development of rat spindles is dependent upon sensory but not motor innervation. Moreover, afferents might regulate the gene expression responsible for synthesis of myosins isoforms specific to intrafusal fibers only in those myonuclei located within the capsule, but not in the myonuclei in extracapsular regions of intrafusal fibers.
Similar content being viewed by others
References
Bader D, Masaki T, Fischman DA (1982) Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol 95:763–770
Banks RW, Harker DW, Stacey MJ (1977) A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique. J Anat 123:783–796
Boyd IA, Gladden MH (1985) Morphology of mammalian muscle spindles, a review. In: Boyd IA, Gladden MH (eds) The muscle spindle. Macmillan, London, pp 3–22
Draeger A, Weeds AG, Fitzsimons RB (1987) Primary, secondary, and tertiary myotubes in developing skeletal muscle: a new approach to the analysis of human myogenesis. J Neurol Sci 81:19–43
Guth L, Samaha FJ (1970) Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28:365–367
Hess A (1970) Vertebrate slow muscle fibers. Physiol Rev 50:40–62
te Kronnie G, Donselaar Y, Soukup T, van Raamsdonk W (1981) Immunohistochemical differences in myosin composition among intrafusal muscle fibers. Histochemistry 73:65–74
te Kronnie G, Donselaar Y, Soukup T, Zelena J (1982) Development of immunohistochemical characteristics of intrafusal fibres in normal and de-efferented rat muscle spindles. Histochemistry 74:355–366
Kucera J (1977) Histochemistry of intrafusal muscle fibers outside the spindle capsule. Am J Anat 148:427–432
Kucera J (1980) Myofibrillar ATPase activity of intrafusal fibers in chronically deafferented rat muscle spindles. Histochemistry 66:221–228
Kucera J, Walro JM (1987) Postnatal maturation of spindles in deafferented rat soleus muscles. Anat Embryol 176:449–461
Kucera J, Walro JM (1988a) The effect of neonatal deafferentation or deefferentation on myosin expression in intrafusal muscle fibers of the rat. Histochemistry (in press)
Kucera J, Walro JM (1988b) Density and distribution of spindles in developing soleus muscles of the rat. J Physiol London 401:78P
Kucera J, Dorovini-Zis K, Engel WK (1978) Histochemistry of rat intrafusal muscle fibers and their motor innervation. J Histochem Cytochem 26:971–988
Kucera J, Walro JM, Reichler J (1988a) Motor and sensory innervation of muscle spindles in the neonatal rat. Anat Embryol 177:427–436
Kucera J, Walro JM, Reichler J (1988b) Innervation of developing intrafusal muscle fibers in the rat. Am J Anat (in press)
Maier A, Gambke B, Pette D (1988) Immunohistochemical demonstration of embryonic myosin in adult mammalian intrafusal fibers. Histochemistry 88:267–271
Milburn A (1973a) The early development of muscle spindles in the rat. J Cell Sci 12:175–195
Milburn A (1973b) The development of the muscle spindle in the rat. Ph D thesis, Durham University
Narusawa M, Fitzsimons RB, Izumo S, Nadal-Ginard B, Rubinstein NA, Kelly AM (1987) Slow myosin in developing rat skeletal muscle. J Cell Biol 104:447–459
Ovalle WK, Smith RS (1972) Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol 50:195–202
Pierobon-Bormioli S, Sartore S, Vitadello M, Schiaffino S (1980) Slow myosins in vertebrate skeletal muscle. An immunofluorescence study. J Cell Biol 85:672–681
Rowlerson A, Gorza L, Schiaffino S (1985) Immunohistochemical identification of spindle fibre types in mammalian muscle using type-specific antibodies to isoforms of myosin. In: Boyd IA, Gladden MH (eds) The muscle spindle. Macmillan, London, pp 29–34
Shafiq SA, Shimuzu T, Fischman DA (1984) Heterogeneity of type I skeletal muscle fibers revealed by monoclonal antibody to slow myosin. Muscle Nerve 7:380–387
Smith RS (1966) Properties of intrafusal muscle fibers. In: Muscle afferents and motor control. Almquist and Wikesell, Stockholm, pp 60–80
Stockdale FE, Miller JB (1987) The cellular basis of myosin heavy chain isoform expression during development of avian skeletal muscles. Dev Biol 123:1–9
Tower SS (1932) Atrophy and degeneration of the muscle spindle. Brain 55:77–90
Walro JM, Kucera J (1985a) Motor innervation of intrafusal fibers in rat muscle spindles: incomplete separation of dynamic and static systems. Am J Anat 173:55–68
Walro JM, Kucera J (1985b) Rat muscles deficient in elements of the γ static system. Neurosci Lett 59:303–307
Zelena J (1957) The morphogenetic influence of innervation on the ontogenic development of muscle spindles. J Embryol Exp Morphol 5:283–292
Zelena J (1976) The role of sensory innervation in the development of mechanoreceptors. Prog Brain Res 43:49–64
Zelena J, Soukup T (1973) Development of muscle spindles deprived of fusimotor innervation. Z Zellforsch Mikrosk Anat 144:435–452
Zelena J, Soukup T (1974) The differentiation of intrafusal fibre types in rat muscle spindles after motor denervation. Cell Tissue Res 153:115–136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kucera, J., Walro, J.M. Postnatal expression of myosin heavy chains in muscle spindles of the rat. Anat Embryol 179, 369–376 (1989). https://doi.org/10.1007/BF00305063
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305063