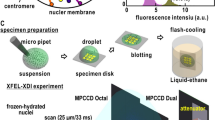
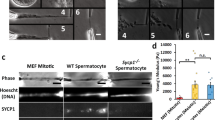
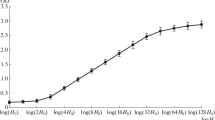
Abstract
Measurements of viscoelastic retardation times of detergent-Pronase lysates of Drosophila cells demonstrated the presence of large numbers of DNA molecules of a size commensurate with that of the chromosomes. The values estimated from the retardation times for the molecular weights of the largest molecules ranged from about 20×109 to 80×109 daltons depending on the species of Drosophila. The molecular weights of the DNA molecules were independent of the metaphase shapes (i.e., metacentric or submetacentric), but were proportional to the DNA contents of the chromosomes in the case of translocations or deletions. It was concluded, therefore, that the DNA molecules must run the length of the chromosome and cannot be discontinuous at the centromere. When compared with the values of the DNA contents of Drosophila chromosomes determined by other methods, the results were consistent with the model of one, or possibly two, DNA molecules per chromosome; the simplest conclusion, that there is only one DNA molecule per chromosome (for simple chromosomes), rests on a long extrapolation of an empirical relation between retardation time and molecular weight, but is also favored by indirect evidence. Further possibilities which could not be excluded were that the large DNA molecules contained Pronase-resistant, non-DNA links, or that a fraction of smaller DNA molecules might also have been present in the chromosomes. Chromosome-sized DNA molecules were obtained almost quantitatively from unsynchronized cultured cells, suggesting that the size of the chromosomal DNA is conserved throughout much of the cell cycle. The molecules were stable for periods of up to several days at 50° C in solutions containing detergent, Pronase, and EDTA.
Similar content being viewed by others
References
Alberts, B.M., Amodio, F.J., Jenkins, M., Guttman, E.D., Ferris, F.L.: Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spr. Harb. Symp. quant. Biol. 33, 289–305 (1968).
Bendich, A., Rosenkranz, H.: Some thoughts on the double-stranded model of deoxyribonucleic acid. Progress in nucleic acid research (J.N. Davidson and W.E. Cohn, eds.) vol. 1, p. 219–230. New York: Academic Press 1963.
Blair, D.G., Sheratt, D.J., Clewell, D.B., Helinski, D.R.: RNase and alkali sensitive supercoiled Col E1 DNA synthesized in E. coli in the presence of chloramphenicol. Fed. Proc. 31, 442, abstr. No. 1269 (1972).
Cairns, J.: A minimum estimate for the length of the DNA of Escherichia coli obtained by autoradiography. J. molec. Biol. 4, 407–409 (1962).
Cairns, J.: The bacterial chromosome and its manner of replication as seen by autoradiography. J. molec. Biol. 6, 208–213 (1963).
Cairns, J.: Autoradiography of HeLa cell DNA. J. molec. Biol. 15, 372–373 (1966).
Callan, H.G., MacGregor, H.C.: Action of deoxyribonuclease on lampbrush chromosomes. Nature (Lond.) 181, 1479–1480 (1958).
Chapman, R.E., Jr., Klotz, L.C., Thompson, D.S., Zimm, B.H.: An instrument for measuring retardation times of deoxyribonucleic acid solutions. Macromolecules 2, 637–643 (1969).
Clewell, D.B., Helinski, D.R.: Supercoiled circular DNA-protein complex in Escherichia coli: Purification and induced conversion to an open circular DNA form. Proc. nat. Acad. Sci. (Wash.) 62, 1159–1166 (1969).
Davison, P.F.: The effect of hydrodynamic shear on the deoxyribonucleic acid from T2 and T4 bacteriophages. Proc. nat. Acad. Sci. (Wash.) 45, 1560–1568 (1959).
Davison, P.F., Freifelder, D., Hede, R., Levinthal, C.: The structural unit of the DNA of T2 bacteriophage. Proc. nat. Acad. Sci. (Wash.) 47, 1123–1129 (1961).
Dickson, E., Boyd, J., Laird, C.: Sequence diversity of polytene chromosome DNA from Drosophila hydei. J. molec. Biol. 61, 615–627 (1971).
Dounce, A.L.: Nuclear gels and chromosomal structure. Amer. Scientist 59, 74–83 (1971).
Dounce, A.L.: Role of enzymes in the isolation of deoxyribonucleic acid with sodium dodecyl sulfate. Arch. Biochem. Biophys. 111, 506–519 (1965).
DuPraw, E.J.: DNA and chromosomes. New York: Holt, Rinehart and Winston, Inc. 1970.
Errera, M.: In vitro and in situ action of ionizing radiations on nucleo-proteins of the cell nucleus. Cold Spr. Harb. Symp. quant. Biol. 12, 60–63 (1947).
Fredericq, E.: Gel-forming properties of thymus nucleoproteins. Biochim. biophys. Acta (Amst.) 55, 300–309 (1962).
Freifelder, D.: Molecular weights of coliphages and coliphage DNA. J. molec. Biol. 54, 567–577 (1970).
Gay, H., Das, C.C., Forward, K., Kaufmann, B.P.: DNA content of mitotically active condensed chromosomes of Drosophila melanogaster. Chromosoma (Berl.) 32, 213–223 (1970).
Guggenheim, E.A.: On the determination of the velocity constant of a unimolecular reaction. Phil. Mag. and J. of Science, Ser. 7 2, 538–543 (1926).
Hays, J.B., Zimm, B.H.: Flexibility and stiffness in nicked DNA. J. molec. Biol. 48, 297–317 (1970).
Hennig, W.: Highly repetitive DNA sequences in the genome of Drosophila hydei. I. Preferential localization in the X chromosomal heterochromatin. J. molec. Biol. (in press, 1972).
Hotta, Y., Bassel, A.: Molecular size and circularity of DNA in cells of mammals and higher plants. Proc. nat. Acad. Sci. (Wash.) 53, 356–362 (1965).
Hotta, Y., Stern, H.: A DNA-binding protein in meiotic cells of Lilium. Develop. Biol. 26, 87–99 (1971).
Huberman, J.A., Riggs, A.D.: Autoradiography of chromosomal DNA fibers from Chinese hamster cells. Proc. nat. Acad. Sci. (Wash.) 55, 599–606 (1966).
Huberman, J.A., Riggs, A.D.: On the mechanism of DNA replication in mammalian chromosomes. J. molec. Biol. 32, 327–341 (1968).
Kaufmann, B.P.: Somatic mitoses of Drosophila melanogaster. J. Morph. 56, 125–155 (1934).
Kavenoff, R.: Sedimentation measurements of bacterial DNA molecules. Ph.D. Thesis, Albert Einstein, College of Medicin, N.Y. (1971).
Kavenoff, R.: Characterization of the Bacillus subtilis W23 genome by sedimentation. J. molec. Biol. (in press, 1972).
Klotz, L.C., Zimm, B.H.: Retardation times of deoxyribonucleic acid solutions. II. Improvement in apparatus and theory. Macromolecules 5, 471 (1972a).
Klotz, L.C., Zimm, B.H.: Size of DNA determined by viscoelastic measurements: Results on bacteriophages, B. subtilis and E. coli. J. molec. Biol. (in press, 1972b).
Kram, R., Botchan, M., Hearst, J.E.: Arrangement of the highly reiterated DNA sequences in the centric heterochromatin of Drosophila melanogaster evidence for interspersed spacer DNA. J. molec. Biol. 64, 103–117 (1972).
Laird, C.D.: Chromatid structure: Relationship between DNA content and nucleotide sequence diversity. Chromosoma (Berl.) 32, 378–416 (1971).
Levin, D., Hutchinson, F.: Neutral sucrose sedimentation of very large DNA from Bacillus subtilis: I. Effect of random double-strand breaks and centrifuge speed on sedimentation. J. molec. Biol. (in press, 1972).
Lindsley, D.L., Grell, E.H.: Genetic variations of Drosophila melanogaster. Washington: Carnegie Institution of Washington 1968.
Meselson, M., Stahl, F.W.: The replication of DNA in Escherichia coli. Proc. nat. Acad. Sci. (Wash.) 44, 671–682 (1958).
Mulder, M.P., Duijn, P. van, Gloor, H.J.: The replicative organization of DNA in polytene chromosomes of Drosophila hydei. Genetica ('s-Gravenhage) 39, 385–428 (1968).
Patterson, J.T., Stone, W.S.: Evolution in the genus Drosophila. New York: Macmillan 1952.
Plaut, W., Nash, D., Fanning, T.: Ordered replication of DNA in polytene chromosomes of Drosophila melanogaster. J. molec. Biol. 16, 85–93 (1966).
Rasch, E.M., Barr, H.J., Rasch, R.W.: The DNA content of sperm of Drosophila melanogaster. Chromosoma (Berl.) 33, 1–18 (1971).
Ris, H., Kubai, D.F.: Chromosome structure. Ann Rev. Genet. 4, 263–294 (1970).
Rolfe, R.: The molecular arrangement of the conserved subunits of DNA. J. molec. Biol. 4, 22–30 (1962).
Rosenberg, A.H., Studier, F.W.: Intrinsic viscosity of native and single-stranded T7 DNA and its relationship to sedimentation coefficient. Biopolymers 1, 765–774 (1969).
Ross, P.D., Scruggs, R.L.: Viscosity study of DNA II. The effect of simple salt concentration on the viscosity of high molecular weight DNA and application of viscometry to the study of DNA isolated from T4 and T5 bacteriophage mutants. Biopolymers 6, 1005–1018 (1968).
Rubenstein, I., Leighton, S.B.: The influence of rotor speed on the sedimentation behavior in sucrose gradients of high molecular weight DNA's. Biophys. Soc., Abstr. 209a (1971).
Rudkin, G.T.: The structure and function of heterochromatin. In: Genetics today. Proc. XIth Intern. Cong. Genet. vol. 2, p. 359–374. New York: Pergamon Press 1964.
Rudkin, G.T.: Photometric measurements of individual metaphase chromosomes. In Vitro 1, 12–20 (1965).
Schneider, I.: Differentiation of larval Drosophila eye-antennal discs in vitro. J. exp. Zool. 156, 91–104 (1964).
Schneider, J.: Cell lines of Drosophila melanogaster. J. Embryol. exp. Morph. 27, 353–365 (1972).
Scruggs, R.L., Ross, P.D.: Viscosity study of DNA. Biopolymers 2, 593–609 (1964).
Taylor, J.H., Woods, P.S., Hughes, W.T.: The organization and duplication of chromosomes as revealed by autoradiographic studies using tritium-labeled thymidine. Proc. nat. Acad. Sci. (Wash.) 43, 122–128 (1957).
Trosko, J.E., Wolff, W.: Strandedness of vicia faba chromosomes as revealed by enzyme digestion studies. J. Cell Biol. 26, 125–135 (1965).
Watson, J.D., Crick, F.H.C.: The structure of DNA. Cold Spr. Harb. Symp. quant. Biol. 18, 123–131 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kavenoff, R., Zimm, B.H. Chromosome-sized DNA molecules from Drosophila . Chromosoma 41, 1–27 (1973). https://doi.org/10.1007/BF00284071
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00284071