Summary
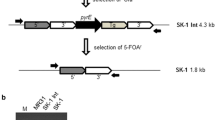
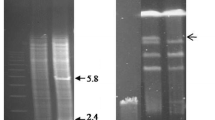
A broad-spectrum mercury resistance locus (mer) from a spontaneous chloramphenicol-sensitive (Cms), arginine auxotrophic (Arg−) mutant of Streptomyces lividan 1326 was isolated on a 6 kb DNA fragment by shotgun cloning into the mercury-sensitive derivative S. lividans TK64 using the vector pIJ702. The mer genes form part of a very large amplifiable DNA sequence present in S. lividans 1326. This element was amplified to about 20 copies per chromosome in the Cms Arg− mutant and was missing from strains like S. lividans TK64, cured for the plasmid SLP3. DNA sequence analysis of a 5 kb region encompassing the whole region required for broad-spectrum mercury resistance revealed six open reading frames (ORFs) transcribed in opposite directions from a common intercistronic region. The protein sequences predicted from the two ORFs transcribed in one direction showed a high degree of similarity to mercuric reductase and organomercurial lyase from other gram-negative and gram-positive sources. Few, if any, similarities were found between the predicted polypeptide sequences of the other four ORFs and other known proteins.
Similar content being viewed by others
References
Altenbuchner J, Cullum J (1985) Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet 201:192–197
Babich K, Engle JM, Skinner JS, Laddaga RA (1991) Deletion mutant analysis of the Staphylococcus aureus plasmid pI258 mercury resistance determinant. Canad J Microbiol 37:624–631
Bernan V, Filpula D, Herber W, Bibb MJ, Katz E (1985) The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterisation of the gene product. Gene 37:101–110
Bibb MJ, Findlay PR, Johnson MW (1984) The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30:157–166
Bibb MJ, Ward JH, Cohen SN (1985) Nucleotide sequences encoding and promoting expression of three antibiotic resistance genes indigenous to Streptomyces. Mol Gen Genet 199:26–39
Bibb MJ (1986) Gene expression in Streptomyces: nucleotide sequences involved in initiation of transcription and translation. In: Szabó G, Biro S, Goodfellow M (eds) Biological, biochemical and biomedical aspects of Actinomycetes, Part A. Akadémiai Kiadó, Budapest
Bogdanova ES, Mindlin SZ (1989) Two structural types of mercury reductases and possible ways of their evolution. FEBS Lett 247:333–336
Brown NL, Ford SJ, Pridmore RD, Fritzinger DC (1983) Nucleotide sequence of a gene from the Pseudomonas transposon TN501 encoding mercuric reductase. Biochemistry 22:4089–4095
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Eichenseer C, Altenbuchner J (1991) Amplifiable elements of Streptomyces lividans are useful tools to increase gene expression. In: Reuss M, Chmiel H, Gilles E-D, Knackmuss H-J (eds) Biochemical engineering-Stuttgart, Gustav Fischer, Stuttgart
Fox BS, Walsh CT (1983) Mercuric reductase: homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry 22:4082–4088
Gambill BD, Summers AO (1985) Versatile mercury-resistant cloning and expression vectors. Gene 39:293–297
Gribskov M, Devereux J, Burgess RR (1984) The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res 12:539–549
Griffin HG, Foster TJ, Silver S, Misra TK (1987) Cloning and DNA sequence of the mercuric and organomercurial resistance determinants of plasmid pDU1358. Proc Natl Acad Sci USA 84:3112–3116
Henderson G, Krygsman P, Jun Liu CI, Davey CC, Malek LT (1987) Characterization and structure of genes for proteases A and B from Streptomyces griseus. J Bacteriol 169:3778–3784
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197
Hopwood DA, Kieser TR, Wright HM, Bibb MJ (1983) Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol 129:2257–2269
Hopwood DA, Lydiate DJ, Malpartida F, Wright HM (1984) Conjugative sex plasmids in Streptomyces. In: Helinski D, Cohen S, Clewell D (eds) Plasmids in bacteria. Plenum Press, New York, pp 615–634
Hopwood DA, Bibb MJ, Chater RF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: A laboratory manual. John Innes Foundation, Norwich
Hussain M, Pastor FIJ, Lampen JO (1987) Cloning and sequencing of the blaZ gene encoding β-lactamase III, a lipoprotein of Bacillus cereus 569/H. J Bacteriol 169:579–586
Katz E, Thompson CJ, Hopwood DA (1983) Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714
Kieser T (1984) Factors affecting the isolation of ccc-DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36
Kusano T, Jl G, Inoue C, Silver S (1990) Constitutive synthesis of a transport function encoded by the Thiobacillus ferrooxidans merC gene cloned in Escherichia coli. J Bacteriol 172:2688–2692
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. Nucleic Acids Res 157:105–132
Laddaga RA, Chu L, Misra TK, Silver S (1987) Nucleotide sequence and expression of the mercurial resistance operon from Staphylococcus aureus plasmid p1258. Proc Natl Acad Sci USA 84:5106–5110
Marsh LJ, Erfle M, Wykes EJ (1984) The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32:481–485
McKenney R, Shimatake H, Court D, Schmeissner U, Braty C, Rosenberg M (1981) Analysis of nucleic acids by enzymatic methods. In: Chirikyian JG, Papas TS (eds), Gene amplification and analysis, Vol. 2, Elsevier North-Holland, Amsterdam, pp 383–415
Misra TK (1992) Bacterial resistances to inorganic mercury salts and organomercurials. Plasmid 27:4–16
Misra TK, Brown N, Haberstroh L, Schmidt A, Godette D, Silver S (1985) Mercuric reductase structural genes from plasmid R100 and transposon Tn501: functional domains of the enzyme. Gene 34:253–262
Nakahara H, Silver S, Miki T, Rownd RH (1979) Hypersensitivity to Hg2+ and hyperbinding activity associated with cloned fragments of the mercurial resistance operon of plasmid NR1. J Bacteriol 140:161–166
Nakahara H, Schottel JL, Yamada T, Miyakawa Y, Asakawa M, Harville J, Silver S (1985) Mercuric reductase enzymes from Streptomyces species and Group B Streptococcus. J Gen Microbiol 131:1053–1059
O'Halloran T, Frantz B, Shin MK, Ralston DM, Wright JC (1989) The merR metal receptor mediates positive activation in a topologically novel transcription complex. Cell 56:119–129
Polsinelli M, Beretta M (1966) Genetic recombination in crosses between Streptomyces aureofaciens and Streptomyces rimosus. J Bacteriol 91:63–68
Robbins PW, Trimble RB, Wirth DF, Hering F, Maley GF, Maley RDas, Gibson BW, Royal N, Bieman K (1984) Primary structure of the Streptomyces enzyme endo-β-N-acetylglucosaminidase H. J Biol Chem 259:7577–7583
Rosenstein R, Peschel A, Wieland B, Götz F (1992) Expression and regulation of the antimony, arsenite and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol 174:3676–3683
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
San Francisco MDJ, Hope CL, Owolabi JB, Tisa LS, Rosen BP (1990) Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res 18:619–624
Silver S, Misra TK, Laddaga A (1989) DNA sequence analysis of bacterial toxic heavy metal resistances. Biol Trace Element Res 21:145–163
Strohl WR (1922) Compilation and analysis of DNA sequences associated with streptomycete promoters. Nucleic Acids Res 20:961–974
Tautz D, Renz M (1983) An optimized freeze-squeeze methode for the recovery of DNA fragments from agarose gels. Anal Biochem 132:14–19
Tohyama H, Okami Y, Umezawa H. (1987) Nucleotide sequence of the streptomycinphosphotransferase and amidonotransferase genes from Streptomyces griseus. Nucl Acids Res 15:1819–1833
van Heijne G (1989) The structure of signal peptides from bacterial lipoproteins. Protein Eng 2:531–534
Wang Y, Moore M, Levinson HS, Silver S, Walsh C, Mahler I (1989) Nucleotide sequence of a chromosomal mercury resistance determinant from a Bacillus sp. with broad-spectrum mercury resistance. J Bacteriol 171:83–92
Walsh CT, Distefano MD, Moore MJ, Shewchuk LM, Verdine GL (1988) Molecular basis of bacterial resistance to organomercurial and inorganic mercury salts. FEBS 2:124–130
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103–119
Zagursky R, Baumeister K, Lomax N, Berman M (1986) Dideoxy DNA sequencing directly from plasmid DNA using reverse transcriptase. Gene Anal Tech 2:89–95
Author information
Authors and Affiliations
Additional information
Communicated by J. Lengeler
Rights and permissions
About this article
Cite this article
Sedlmeier, R., Altenbuchner, J. Cloning and DNA sequence analysis of the mercury resistance genes of Streptomyces lividans . Molec. Gen. Genet. 236, 76–85 (1992). https://doi.org/10.1007/BF00279645
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279645