Summary
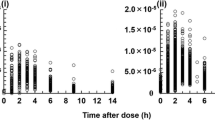
The intraindividual variability and circadian variation of oral cyclosporine (CsA) pharmacokinetics were studied over 24 h in 18 renal transplant recipients at steady state, and in 10 of the patients during a second 24 h period.
The absolute percentage intraindividual difference in daytime AUC (0–12 h) ranged from 2% to 54% (mean 30%), and the corresponding variability in nighttime AUC (0–12 h) ranged from 5% to 80% (mean 34%). The pharmacokinetic variables t1/2, tmax and Cmax were more variable than the AUC (0–12 h) both during the day and at night. The evening trough level was significantly lower than the morning trough level; 185 ng · ml−1 versus 223 ng · ml−1. This, together with a significantly longer t1/2 in the night than the day, suggested circadian variability in the pharmacokinetics of CsA.
In a separate retrospective study in 162 renal transplant recipients given CsA by constant intravenous infusion, repeated CsA blood concentration measurements at steady state showed lower concentrations during the day than the night, suggesting higher CsA clearance during daytime.
It is concluded that CsA pharmacokinetics in renal transplant recipients, besides the well-known interindividual variability, also displays large intraindividual variability as well as circadian variation. Our findings further emphasize the necessity and difficulty of pharmacological monitoring in the clinical use of CsA in organ transplantation.
Similar content being viewed by others

References
European Multicenter Trial Group (1983) Cyclosporin in cadaver renal transplantation: one-year follow-up of a multicentre trial. Lancet II: 986–989
Canadian Multicentre Transplant Study Group (1983) A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med 309: 809–815
Shaw LM, Bowers L, Demers L, Freeman D, Moyer T, Sanghvi A, Seltman H, Venkataramanan R (1987) Critical issues in cyclosporine monitoring: report of the task force on cyclosporine monitoring. Clin Chem 33: 1269–1288
Klintmalm G, Säwe J, Ringdén O, von Bahr C, Magnusson A (1985) Cyclosporine plasma levels in renal transplant patients: association with renal toxicity and allograft rejection. Transplantation 39: 132–137
Lindholm A (1991) Therapeutic monitoring of cyclosporin — an update. Eur J Clin Pharmacol 41: 273–283
Ptachcinski RJ, Venkataramanan R, Burckart GJ (1986) Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet 11: 107–132
Lindholm A (1991) Factors influencing the pharmacokinetics of cyclosporine in man. Ther Drug Monit 13: 465–477
Bowers LD, Canafax DM, Sing J, Seifedlin R, Simmons RL, Najarian JS (1986) Studies of cyclosporine blood levels: analysis, clinical utility, pharmacokinetics, metabolites, and chronopharmacology. Transplant Proc 18 [Suppl 5]: 137–143
Sabaté I, Griñó JM, Castelao AM, Arranz B, Gonzalez C, Guillén E, Diaz C, Huguet J, Gracia S (1990) Diurnal variations of cyclosporine and metabolites in renal transplant patients. Transplant Proc 22: 1700–1701
Ramon M, Morel D, Penouil F, Grellet J, Potaux L, Saux MC, Brachet-Liermain A (1989) Variations nycthémérales de la ciclosporine administrée par voie orale á des transplantés rénaux. Therapie 44: 371–374
Lindholm A, Henricsson S, Lind M, Dahlqvist R (1988) Intraindividual variability in relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol 34: 461–464
Kahan BD, Grevel J (1988) Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation 46: 631–644
Kahan BD, Welsh M, Rutzky L, Lewis R, Knight R, Katz S, Napoli K, Grevel J, van Buren CT (1992) The ability of pretransplant test dose pharmacokinetic profiles to reduce early adverse events after renal transplantation. Transplantation 53: 345–351
Gupta SK, Legg B, Solomon LR, Johnson RWG, Rowland M (1987) Pharmacokinetics of cyclosporin: influence of rate of constant intravenous infusion in renal transplant patients. Br J Clin Pharmacol 24: 519–526
Lindholm A, Henricsson S (1990) Comparative analyses of cyclosporine in whole blood and plasma by radioimmunoassay, fluorescence polarization immunoassay and high pressure liquid chromatography. Ther Drug Monit 12: 344–352
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR (1985) Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther 38: 296–300
Burckart GJ, Venkataramanan R, Ptachcinski RJ, Starzl TE, Gartner JC Jr, Zitelli BJ, Malatack JJ, Shaw BW, Iwatsuki S, van Thiel DH (1986) Cyclosporine absorption following orthotopic liver transplantation. J Clin Pharmacol 26: 647–651
Frey FJ, Horber FF, Frey BM (1988) Trough levels and concentration time curves of cyclosporine in patients undergoing renal transplantation. Clin Pharmacol Ther 43: 55–62
Venkataramanan R, Yang S, Burckart GJ, Ptachcinski RJ, van Thiel DH, Starzl TE (1986) Diurnal variation in cyclosporine kinetics. Ther Drug Monit 8: 380–381
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohlman, S., Lindholm, A., Hägglund, H. et al. On the intraindividual variability and chronobiology of cyclosporine pharmacokinetics in renal transplantation. Eur J Clin Pharmacol 44, 265–269 (1993). https://doi.org/10.1007/BF00271369
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00271369