Summary
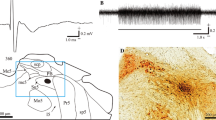
Primary afferent and descending corticobulbar convergence on 186 interneurones located in the intertrigeminal area was investigated. The experiments were performed on cats anaesthetized with chloralose. Nerves from the three trigeminal dermatomes were stimulated electrically at intensities below and above twice the threshold level. Nerves from oral, perioral and periorbital structures, and afferents from the masseteric and digastric muscles were included. The surface of the cerebral cortex was stimulated electrically in systematically selected, maximally receptive points within the trigeminal primary projection fields. The intertrigeminal neurones generally responded to stimulation of lowthreshold afferents from periodontal, lingual or perioral cutaneous receptors with a polysynaptic latency. Inputs from 3–5 nerves were common but one afferent input was usually most effective. The neurones were generally discharged from two or more cortical points, as a rule those of the oral and perioral projection fields in areas 3a and 3b of the coronal gyrus. The fastest path from the cerebral cortex to the intertrigeminal area was monosynaptic. However, the median latency was 4–5 ms which indicates an oligosynaptic path. The path went through the pyramide at the pontine level. The discharge pattern of the intertrigeminal neurones was 1–4 spikes in 54% of the neurones and a high frequency train of spikes in 46%. Cortical excitation followed by inhibition of the neurones was observed. The neurones were not discharged by electrical stimulation in the defence-attack area of the hypothalamus. Transsynaptic responses evoked from the mesencephalon were seen in 1/3 of the tested neurones.
Similar content being viewed by others
Abbreviations
- Alv inf:
-
Inferior alveolar nerve
- Co:
-
Contralateral
- Cx:
-
Cortex cerebri
- Dig:
-
Digastric nerve
- Ip:
-
Ipsilateral
- Ling:
-
Lingual nerve
- Mass:
-
Masseteric nerve
- Mb:
-
Medial borderzone of NVmt
- Mx-w:
-
Infraorbital nerve, a branch to whiskers and lip
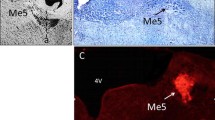
- NintV:
-
Intertrigeminal area
- NVmt:
-
Trigeminal motor nucleus
- NsV:
-
Supratrigeminal nucleus (area)
- NVsnpr-d:
-
Main sensory trigeminal nucleus, dorsal division
- NVsnpr-v:
-
Main sensory trigeminal nucleus, ventral division
- Opht:
-
Ophtalmic nerve
- Vb:
-
Ventral borderzone of NVmt
References
Alstermark B, Lundberg A, Norrsell U. Sybirska E (1981) Integration in descending motor pathways controlling the forelimb in the cat. Exp Brain Res 42: 299–318
Alstermark B, Pinter M, Sasaki S (1983) Convergence on reticulospinal neurons mediating contralateral pyramidal disynaptic EPSP's to neck motoneurons. Brain Res 259: 151–154
Alstermark B, Pinter M, Sasaki S (1985) Pyramidal effects in dorsal neck motoneurones of the cat. J Physiol (Lond) 363: 287–302
Brodal A, Szabo T, Torvik A (1956) Corticofugal fibres to sensory trigeminal nuclei and nucleus of solitary tract. An experimental study in the cat. J Comp Neurol 106: 527–555
Dubner R (1967) Interaction of peripheral and central input in the main sensory nucleus of the cat. Exp Neurology 17: 186–202
Eisenman J, Landgren S, Novin D (1963) Functional organization in the main sensory trigeminal nucleus and in the rostral subdivisions of the nucleus of the spinal trigeminal tract in the cat. Acta Physiol Scand Suppl 59: 214, pp 1–44
Eisenman J, Fromm G, Landgren S, Novin D (1964) The ascending projections of trigeminal neurones in the cat, investigated by antidromic stimulation. Acta Physiol Scand 60: 337–350
Gobel S (1971) Structural organization in the main sensory trigeminal nucleus. In: Dubner R, Kawamura Y (eds) Oralfacial sensory and motor mechanisms. Appelton-CenturyCrofts, New York, pp 183–204
Goldberg LJ, Nakamura Y (1968) Lingually induced inhibition of masseteric motoneurons. Experimentia 24: 371–373
Gordon G, Landgren S, Seed W (1961) The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol (Lond) 158: 544–559
Gura EV, Limansky Yu P, Piliavsky AJ (1972) Functional properties of interneurones in the masticatory reflex. Nejrofiziologija 4: 150–157
Grant G, Landgren S, Silfvenius H (1975) Columnar distribution of U-fibres from the postcruciate cerebral projection area of the cat's group I muscle afferents. Exp Brain Res 24: 57–74
Harrison PJ, Jankowska E (1985) Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol (Lond) 361: 379–401
Hassler R, Muhs-Clement K (1964) Architektonischer Aufbau des sensomotorischen und parietalen Cortex der Katze. J Hirnforsch 6: 377–420
Hultborn H, Illert M, Santini M (1976) Convergence on interneurons mediating the reciprocal Ia inhibition of motoneurones. Acta Physiol Scand 96: 351–367
Illert M, Lundberg A, Tanaka R (1976a) Integration in descending motor pathways controlling the forelimb in the cat. I. Pyramidal effects on motoneurones. Exp Brain Res 26: 509–519
Illert M, Lundberg A, Tanaka R (1976b) Integration in descending motor pathways controlling the forelimb in the cat. II. Convergence on neurons mediating disynaptic cortico-motoneuronal excitation. Exp Brain Res 26: 521–540
Illert M, Lundberg A, Tanaka R (1977) Integration in descending motor pathways controlling the forelimb in the cat. III. Convergence on propriospinal neurones transmitting disynaptic excitation from the cortico-spinal tract and other descending tracts. Exp Brain Res 29: 323–346
Jerge CR (1963) The function of the nucleus supratrigeminalis. J Neurophysiol 26: 393–402
Katoh M-E, Taira M, Katakura N, Nakamura Y (1982) Cortically induced effects on trigeminal motoneurones after transection of the brainstem at the pontobulbar junction in the cat. Neurosci Lett 33: 141–146
Kidokoro Y, Kubota K, Shuto S, Sumino R (1968a) Reflex organization of cat masticatory muscles. J Neurophysiol 31: 695–708
Kidokoro Y, Kubota K, Shuto S, Sumino R (1968b) Possible interneurons responsible for reflex inhibition of motoneurons of jaw-closing muscles from the inferior dental nerve. J Neurophysiol 31: 709–716
Kuypers HGJM (1958) An anatomical analysis of cortico-bulbar connexions to the pons and lower brain stem in the cat. J Anat (Lond) 92: 198–218
Kuypers HGJM, Tuerk JD (1964) The distribution of the cortical fibres within the nuclei cuneatus and gracilis in the cat. J Anat (Lond) 98: 143–162
Landgren S, Olsson KÅ (1976) Localization of evoked potentials in the digastric, masseteric, supra- and intertrigeminal subnuclei of the cat. Exp Brain Res 26: 299–318
Landgren S, Olsson KÅ (1980a) Cerebral control of jaw reflexes. In: Andersson D (ed) Physiology, past, present and future. Pergamon Press, Oxford, pp 45–63
Landgren S, Olsson KÅ (1980b) Low threshold afferent projections from the oral cavity and the face to the cerebral cortex of the cat. Exp Brain Res 39: 133–147
Landgren S, Olsson KÅ (1980c) The effect of electrical stimulation in the hypothalamus on the monosynaptic jaw closing and the disynaptic jaw opening reflexes in the cat. Exp Brain Res 39: 389–400
Landgren S, Silfvenius H, Olsson KÅ (1984) The sensorimotor integration in area 3a of the cat. In: Creutzfeldt O, Schmidt RF, Willis WD (eds) Sensory-motor integration in the nervous system. Exp Brain Res Suppl 9: 359–375
Landgren S, Olsson KÅ, Westberg K-G (1986) Bulbar neurones with axon projections to the trigeminal motor nucleus in the cat. Exp Brain Res 65: 98–111
Lorente de Nó R (1922) Contribution al conocimiento del nervo trigemino. In: Ramon y Cajal S (ed) Libro en honor. Jaminez y Molina, Madrid, Vol II, pp 13–30
Lorente de Nó R (1933) Vestibulo-ocular reflex arc. Arch Neurol Psykiat 30: 248–253
Lundberg A, Voorhoeve P (1962) Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand 56: 201–219
Lundberg A, Norrsell U, Voorhoeve P (1962) Pyramidal effects on lumbo-sacral interneurones activated by somatic afferents. Acta Physiol Scand 56: 220–229
Lundberg A (1979a) Multisensory control of spinal reflex pathways. In: Granit R, Pompeiano O (eds) Reflex control of posture and movement, Vol 50. Progr Brain Res, Elsevier, Amsterdam, pp 11–28
Lundberg A (1979b) Integration in a propriospinal motor center controlling the forelimb in the cat. In: Assanuma H, Wilson VJ (eds) Integration in the nervous system. Igaku Shoin, Tokyo New York, pp 47–65
Meessen H, Olszewski J (1949) A cytoarchitectonic atlas of the rombencephalon of the rabbit. S Karger, Basel
Mizuno N, Sauerland EK, Clemente CD (1968) Projection from the orbital gyrus in the cat. I. To brain stem structures. J Comp Neurol 133: 463–476
Mizuno N, Clemente CD, Sauerland EK (1969) Projections from the orbital gyrus in the cat. II. To telecephalic and diencephalic structures. J Comp Neurol 136: 127–142
Mizuno N (1970) Projection fibers from the main sensory trigeminal nucleus and the supratrigeminal region. J Comp Neurol 139: 457–472
Mizuno N, Nomura S, Itoh D, Nakamura Y, Konishi A (1978) Commissural interneurons for masticatory motoneurons: a light and electron microscope study using the horseradish peroxidase tracer technique. Exp Neurol 59: 254–262
Nakamura Y, Mori S, Nagashima H (1973) Origin and central pathways of crossed inhibitory effects of afferents from the masseteric muscle on the masseteric motoneuron of the cat. Brain Res 57: 29–42
Nakamura Y, Nozaki S, Takatori M, Kikuchi M (1976) Possible inhibitory neurons in the bulbar reticular formation involved in the cortically evoked inhibition of the masseteric motoneuron of the cat. Brain Res 115: 512–517
Nakamura Y, Takatori M, Kubo Y, Nozaki S, Enomoto S (1979) Masticatory rhythm formation — facts and a hypothesis. In: Itoh M, Tsukahara K, Kubota K, Yagi K (eds) Integrative control functions of the brain, Vol II. Tokyo Kodansha Scientific, Tokyo, pp 321–331
Nozaki S, Enomoto S, Nakamura Y (1983) Identification and input-output properties of bulbar reticular neurons involved in the cerebral cortical control of trigeminal motoneurons in cats. Exp Brain Res 49: 363–372
Olsson KÅ, Landgren S (1980) Facilitation and inhibition of jaw reflexes evoked by electrical stimulation of the cat's cerebral cortex. Exp Brain Res 39: 149–164
Olsson KÅ, Landgren S, Westberg K-G (1984) Cortical and trigeminal convergence in the intertrigeminal area. Neurosci Lett Suppl 18: S261
Sumino R, Nozaki S, Katoh M (1981) Trigemino-neck reflex. In: Kawamura Y, Dubner R (eds) Oral-facial sensory and motor functions. Quintessence Publ Co, Inc, Tokyo, pp 81–88
Takata M, Kawamura Y (1970) Neurophysiologic properties of the supratrigeminal nucleus. Jpn J Physiol 20: 1–11
Torvik A (1956) Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. An experimental study in the rat. J Comp Neurol 106: 51–142
Walberg F (1957) Do motor nuclei of the cranial nerves receive corticofugal fibres? An experimental study in the cat. Brain 80: 597–605
Wold E, Brodal A (1973) The projection of cortical sensorimotor regions onto the trigeminal nucleus in the cat. An experimental anatomical study. Neurobiology 3: 353–375
Wold E, Brodal A (1974) The cortical projection of the orbital and proreate gyri to the sensory trigeminal nuclei in the cat. An experimental anatomical study. Brain Res 65: 381–395
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Olsson, K.Å., Landgren, S. & Westberg, K.G. Location of, and peripheral convergence on, the interneurone in the disynaptic path from the coronal gyrus of the cerebral cortex to the trigeminal motoneurones in the cat. Exp Brain Res 65, 83–97 (1986). https://doi.org/10.1007/BF00243832
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00243832