Summary
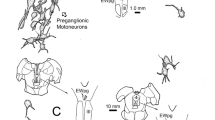
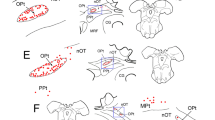
We strictly limited small injection of horseradish peroxidase (HRP) to lamina A of the lateral geniculate nucleus of cats. This was done to label retrogradely only the alpha (Y) and beta (X) classes of retinal ganglion cell. Eighty-six such injections at a range of matched eccentricities were made bilaterally in 9 normal adult cats, 7 cats reared from birth to adulthood with monocular lid suture, and 9 normal kittens at 4 weeks of age; 5348 alpha and beta cells were retrogradely labeled from these injections. Quantitative measurements were made from these labeled cells and compared among 4 experimental conditions, these being normal adult retinas, the nondeprived and deprived retinas of lid sutured cats, and the retinas of kittens. Each injection led to a similar relative ratio of labeled alpha and beta cells (typically 5–15% alpha cells) that did not differ significantly among the experimental conditions, but further analysis suggested a slight dimunition of labeled alpha cells in deprived retinas. Because the larger arbors of retinogeniculate Y axons are more likely to penetrate small geniculate HRP injection sites from eccentric locations than would be the case for the more restricted arbors of X axons, a normal tendency resulted for the peripheral halo of zones of retrograde labeling to be dominated by alpha cells. Thus a more accurate reflection of the relative numbers of labeled alpha and beta cells would result from considering only the core of zones of retrograde labeling. When this is done, deprived retinas exhibited relatively fewer labeled alpha cells than did normal, nondeprived, or kitten retinas. This may relate to prior observations (Sur et al. 1982) that abnormally few Y axons from the derpived retina innervate lamina A. No statistically significant differences in alpha or beta cell size were seen among normal, nondeprived, and deprived retinas, although both of these cell types in the kittens were equally smaller than their normal adult counterparts. This is particularly interesting in view of the postnatal growth of retinogeniculate axon arbors (Sur et al. 1984). The results are not surprising for alpha cells, since retinogeniculate Y axon arbors grow considerably after 4 weeks of age, but they are surprising for beta cells, since retinogeniculate arbors of X axons decrease after 4 weeks of age. This suggests no clear, general relationship between soma size and the extent of a cell's axonal arbor. Overall, these results suggest that no dramatic abnormalities due to rearing with monocular suture are evident at the level of the retina, although subtle effects can be demonstrated there (see also Leventhal and Hirsch 1983). The most peripheral site in the visual system at which such dramatic effects have been documented thus seems to be at the level of retinogeniculate innervation.
Similar content being viewed by others
References
Bishop PO, Kozak W, Vakkur GJ (1962) Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol (Lond) 163: 466–502
Bowling DB, Michael CR (1984) Terminal patterns of single physiologically characterized optic tract fibers in the cat's lateral geniculate nucleus. J Neurosci 4: 198–216
Boycott BB, Wässle H (1974) The morphological types of ganglion cells of the domestic cat's retina. J Physiol (Lond) 240: 397–419
Cleland BG, Mitchell DE, Gillard-Crewther S, Grewther DP (1980) Visual resolution of retinal ganglion cells in monocularly-deprived cats. Brain Res 192: 261–266
Fernald R, Chase R (1971) An improved method for plotting retinal landmarks and focusing the eyes. Vision Res 11: 95–96
Friedlander MJ, Stanford LR, Sherman SM (1982) Effects of monocular deprivation on the structure/function relationship of individual neurons in the cat's lateral geniculate nucleus. J Neurosci 2: 321–330
Fukuda Y, Stone J (1974) Retinal distribution and central projections of Y-, X-, and W-cells of the cat's retina. J Neurophysiol 37: 749–772
Holländer H, Vanegas H (1977) The projection from the lateral geniculate nucleus onto the visual cortex in the cat. A quantitative study with horseradish-peroxidase. J Comp Neurol 173: 519–536
Illing R-B, Wässle H (1981) The retinal projection to the thalamus in the cat: a quantitative investigation and a comparison with the retinotectal pathway. J Comp Neurol 202: 265–285
Kratz KE, Mangel SC, Lehmkuhle S, Sherman SM (1979) Retinal X- and Y-cells in monocularly lid-sutured cats: normality of spatial and temporal patterns. Brain Res 172: 545–551
LeVay S, Wiesel TN, Hubel DH (1980) The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol 191: 1–51
Leventhal AG (1982) Morphology and distribution of retinal ganglion cells projecting to different layers of the dorsal lateral geniculate nucleus in normal and Siamese cats. J Neurosci 2: 1024–1042
Leventhal AG, Hirsch HVB (1983) Effects of visual deprivation upon the morphology of retinal ganglion cells projecting to the dorsal lateral geniculate nucleus of the cat. J Neurosci 3: 332–344
Mesulam M-M, Rosene DL (1979) Sensitivity in horseradish peroxidase neurohisto-chemistry: a comparative and quantitative study of nine methods. J Histochem Cytochem 27: 763–773
Movshon JA, Van Sluyters RC (1981) Visual neural development. Annu Rev Psychol 32: 477–522
deOlmos JS (1977) An improved HRP method for the study of central nervous connections. Exp Brain Res 29: 541–551
Olson CR, Freeman RD (1980) Rescaling of the retinal map of visual space during growth of the kitten's eye. Brain Res 186: 55–65
Rodieck RW, Brening RK (1983) Retinal ganglion cells: properties, types, genera, pathways, and trans-species comparisons. Brain Behav Evol 23: 121–164
Rowe MH, Dreher B (1982) W cell projection to the medial interlaminar nucleus of the cat: implications for ganglion cell classification. J Comp Neurol 204: 117–133
Saito H-A (1983) Morphology of physiologically identified X-, Y-, and W-type ganglion cells of the cat. J Comp Neurol 221: 279–288
Sanderson KJ (1971) The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol 143: 101–118
Sherman SM (1985a) Functional organization of the W-, X-, and Y-cell pathways in the cat: a review and hypothesis. In: Sprágue JM, Epstein AN (eds) Progress in psychobiology and physiological psychology, vol 11. Academic Press, New York, pp 233–314
Sherman SM (1985b) Development of retinal projections to the cat's lateral geniculate nucleus. Trends Neurosci 8: 350–355
Sherman SM, Spear PD (1982) Organization of visual pathways in normal and visually deprived cats. Physiol Rev 62: 738–855
Sherman SM, Stone J (1973) Physiological normality of the retina in visually deprived cats. Brain Res 60: 224–230
Stanford LR, Sherman SM (1984) Structure/function relationships of retinal ganglion cells in the cat. Brain Res 297: 381–386
Stone J, Clarke R (1980) Correlation between soma size and dendritic morphology in cat retinal ganglion cells: evidence of further variation in the γ-cell class. J Comp Neurol 192: 211–217
Stone J, Dreher B, Leventhal A (1979) Hierarchical and parallel mechanisms in the organization of visual cortex. Brain Res Rev 1: 345–394
Sur M, Sherman SM (1982) Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science 218: 389–391
Sur M, Humphrey AL, Sherman SM (1982) Monocular deprivation affects X- and Y-cell retinogeniculate terminations in cats. Nature 300: 183–185
Sur M, Weller RE, Sherman SM (1984) Development of X-, and Y-cell retinogeniculate terminations in kittens. Nature 310: 246–249
Vanegas H, Holländer H, Distel H (1978) Early stages of uptake and transport of horseradish-peroxidase by cortical structures, and its use for the study of local neurons and their processes. J Comp Neurol 177: 193–212
Wässle H (1982) Morphological types and central projections of ganglion cells in the cat retina. In: Osborne N, Chader G (eds) Progress in retinal research. Pergamon, Oxford, pp 125–152
Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26: 1003–1017
Wiesel TN, Hubel DH (1965) Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol 28: 1029–1040
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hsiao, C.F., Sherman, S.M. Alpha and beta cells projecting from retina to lamina A of the lateral geniculate nucleus in normal cats, monocularly deprived cats, and young kittens. Exp Brain Res 61, 413–431 (1986). https://doi.org/10.1007/BF00239530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239530