Abstract
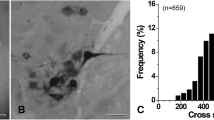
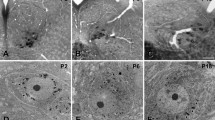
This study has investigated the synaptic interactions between hypoglossal motoneurons and substance P (SP)-immunoreactive terminals. Cholera toxin B conjugated to horseradish peroxidase was injected into the tip of the tongue on the right side of six ketamine-anesthetized cats. Two to five days later, the animals were killed. Cells containing HRP were labeled with a histochemical reaction utilizing tetramethylbenzidine (TMB) as the chromogen. TMB forms crystalline reaction products that are very distinct at the electron microscopic level. The tissues were then processed for immunocytochemisty using an antiserum against SP. The chromogen used in this case, di-aminobenzidine, yields amorphous reaction products. At the light microscopic level, labeled cells were observed primarily ipsilaterally in both intermediate and ventrolateral subdivisions of the hypoglossal nucleus. The majority of these labeled cells were seen at the level of obex. At the electron microscopic level, both asymmetric and symmetric synapses were observed. SP-immunoreactive nerve terminals formed asymmetric synapses with labeled dendrites and symmetric synapses with labeled perikarya. SP-labeled terminals also synapsed on unlabeled dendrites and somata. These are the first ultrastructural studies demonstrating synaptic interactions between hypoglossal motoneurons and SP terminals. These studies demonstrate that hypoglossal motoneurons that innervate intrinsic tongue muscles are modulated by SP and that SP may play a role in the control of fine movements of the tongue.
Similar content being viewed by others
References
Abd-el-Malek S (1938) A contribution to the study of the movements of the tongue in animals, with special reference to the cat. J Anat 73:15–30
Aldes LD, Chronister RB, Marco LA (1988a) Distribution of glutamic acid decarboxylase and gamma-aminobutyric acid in the hypoglossal nucleus in the rat. J Neurosci Res 19:343–348
Aldes LD, Chronister RB, Shelton C, Haycock JW, Marco LA, Wong DL (1988b) Catecholamine innervation of the rat hypoglossal nucleus. Brain Res Bull 21:305–312
Aldes LD, Chronister RC, Marco LA, Haycock JW, Thibault J (1988c) Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp Brain Res 73:305–314
Aldes LD, Marco LA, Chronister RB (1989) Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immuno-electronmicroscopic study. Brain Res Bull 23:249–256
Aldes LD, Shaw B, Chronister RB, Haycock JW (1990) Catechol-amine-containing axon terminals in the hypoglossal nucleus of the rat: an immuno-electronmicroscopic study. Exp Brain Res 81:167–178
Altschuler SM, Bao X, Miselis RR (1994) Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol 342:538–550
Arita H, Sakamoto M, Hirokawa Y, Okado N (1993) Serotonin innervation patterns differ among the various medullary moto-neuronal groups involved in upper airway control. Exp Brain Res 95:100–110
Barnard JW (1940) The hypoglossal complex of vertebrates. J Comp Neurol 72:489–524
Basbaum AI, Clanton CH, Fields HL (1978) Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating mechanisms. J Comp Neurol 178:209–224
Berger AJ, Bayliss DA, Viana F (1992) Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett 143:164–168
Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976) The raphe nuclei of the cat brainstem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113:449–486
Boone TB, Aldes LD (1984) The ultrastructure of two distinct neuron populations in the hypoglossal nucleus of the rat. Exp Brain Res 54:321–326
Borke RC, Nau ME, Ringler RL (1983) Brain stem afferents of hypoglossal neurons in the rat. Brain Res 269:47–55
Chan-Palay V (1982) Immunocytochemical and autoradiographic methods to demonstrate the coexistence of neuroactive substances: cerebellar Purkinje cells have glutamic acid decarboxylase and motilin immunoreactivity, and raphe neurons have serotonin and substance P immunoreactivity. In: Chan-Palay V, Palay SL (eds) Cytochemical methods in neuroanatomy. Liss, New York, pp 93–118
Connaughton M, Priestley JV, Sofroniew MV, Eckenstein NF, Cuello AC (1986) Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocyto-chemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience 17:205–224
Cooper MH (1981) Neurons of the hypoglossal nucleus of the rat. Otolaryngol Head Neck Surg 89:10–15
Cuello AC, Kanazawa (1978) The distribution of substance P immunoreactive fibers in the rat central nervous system. J Comp Neurol 178:129–156
Gatti PJ, Johnson TA, Shirahata M, Massari VJ (1994) Synaptic interactions of substance P immunoreactive (SP) nerve terminals with hypoglossal (N.XII) motoneurons which innervate the intrinsic muscles of the tongue: an electron microscopic dual labeling study. Soc Neurosci Abstr 20:1588
Helke C, Charlton CG, Wiley RG (1985) Suicide transport of ricin demonstrates the presence of substance P receptors on medullary somatic and autonomic motor neurons. Brain Res 328:190–195
Krammer EB, Rath T, Lischka MF (1979) Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res 170:533–537
Kubin L, Tojima H, Davies RO, Pack AI (1992) Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett 139:243–248
Larsson LI (1981) A novel immunocytochemical model system for specificity and sensitivity screening of antisera against multiple antigens. J Histochem Cytochem 29:408–410
Li Y, Takada M, Mizuno N (1993) The sites of origin of serotoninergic afferent fibers in the trigeminal motor, facial and hypoglossal nuclei in the rat. Neurosci Res 17:307–313
Ljungdahl A, Hokfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat. I. Cell bodies and nerve terminals. Neuroscience 3:861–943
Llewellyn-Smith IJ, Pilowsky P, Minson JB (1993) The tungstate stabilized tetramethylbenzidine reaction for light and electron microscopic immunocytochemistry and for revealing biocytin-filled neurons. J Neurosci Methods 26:27–40
Manaker S, Rizio G (1989) Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J Comp Neurol 290:516–526
Manaker S, Tischler LJ (1993) Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol 334:466–476
Manaker S, Tischler LJ, Morrison AR (1992) Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol 322:68–78
Massari VJ, Johnson TA, Llewellyn-Smith IJ, Gatti PJ (1994) Ultrastructural interactions of substance P nerve terminals with negative chronotropic cardio-inhibitory neurons in the nucleus ambiguus. Brain Res 660:275–287
Miyazaki T, Yoshida Y, Hirano M, Shin T, Kanaseki T (1981) Central location of the motoneurons supplying the thyrohyoid and the geniohyoid muscles as demonstrated by horseradish peroxidase method. Brain Res 219:423–427
Morin D, Monteau R, Hilaire G (1992) Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study in the newborn rat. J Physiol (Lond) 451:605–629
Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N (1994) Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347:249–274
Odutola AB (1976) Cell grouping and Golgi architecture of the hypoglossal nucleus of the rat. Exp Neurol 52:356–371
Peters A, Palay SL, Webster HDeF (1990) The fine structure of the nervous system. Oxford, New York, Oxford
Shults CW, Quirion R, Chronwall B, Chase TN, O'Donohue TL (1984) A comparison of the anatomical distribution of substance P and substance P receptors in the rat central nervous system. Peptides 5:1097–1128
Sokoloff AJ (1991) Musculotopic organization of the hypoglossal nucleus in the grass frog, Rana pipiens. J Comp Neurol 308:505–512
Sonntag CF (1925) The comparative anatomy of the tongues of mammalia. XII. Summary, classification and physiology. J Zool Proc Zool Soc Lond 21:701–762
Takada M, Itoh K, Yasui Y, Mitani A, Nomura S, Mizuno N (1984) Distribution of premotor neurons for the hypoglossal nucleus in the cat. Neurosci Lett 52:141–146
Takeuchi Y, Kojima M, Matsuura T, Sano Y (1983) Serotonergic innervation on the motoneurons in the mammalian brainstem. Anat Embryol 167:321–333
Travers JB, Norgren R (1983) Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220:280–298
Uemura M, Matsuda K, Kume M, Takeuchi Y, Matsushima R, Mizuno N (1979) Topographical arrangement of hypoglossal motoneurons: an HRP study in the cat. Neurosci Lett 13:99–104
Uemura-Sumi M, Mizuno N, Nomura S, Iwahori N, Takeuchi Y, Matsushima R (1981) Topographical representation of the hypoglossal nerve branches and tongue muscles in the hypoglossal nucleus of macaque monkeys. Neurosci Lett 22:31–35
Uemura-Sumi M, Itoh M, Mizuno N (1988) The distribution of hypoglossal motoneurons in the dog, rabbit and rat. Anat Embryol (Berl) 177:389–394
Wan XS, Trojanowski JQ, Gonatas JO, Liu CN (1982) Cytoarchitecture of the extranuclear and commissural dendrites of hypo-glossal nucleus neurons as revealed by conjugates of horseradish peroxidase with cholera toxin. Exp Neurol 78:167–175
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gatti, P.J., Coleman, W.C., Shirahata, M. et al. Synaptic interactions of retrogradely labeled hypoglossal motoneurons with substance P-like immunoreactive nerve terminals in the cat: a dual-labeling electron microscopic study. Exp Brain Res 110, 175–182 (1996). https://doi.org/10.1007/BF00228549
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228549