Abstract
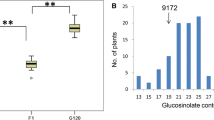
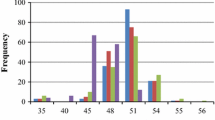
We report the RFLP mapping of quantitative trait loci (QTLs) which regulate the total seed aliphaticglucosinolate content in Brassica napus L. A population of 99 F1-derived doubled-haploid (DH) recombinant lines from a cross between the cultivars Stellar (low-glucosinolate) and Major (high-glucosinolate) was used for singlemarker analysis and the interval mapping of QTLs associated with total seed glucosinolates. Two major loci, GSL-1 and GSL-2, with the largest influence on total seed aliphatic-glucosinolates, were mapped onto LG 20 and LG 1, respectively. Three loci with smaller effects, GSL-3, GSL-4 and GSL-5, were tentatively mapped to LG 18, LG 4 and LG 13, respectively. The QTLs acted in an additive manner and accounted for 71 % of the variation in total seed glucosinolates, with GSL-1 and GSL-2 accounting for 33% and 17%, respectively. The recombinant population had aliphatic-glucosinolate levels of between 6 and 160 μmoles per g-1 dry wt of seed. Transgressive segregation for high seed glucosinolate content was apparent in 25 individuals. These phenotypes possessed Stellar alleles at GSL-3 and Major alleles at the four other GSL loci demonstrating that low-glucosinolate genotypes (i.e. Stellar) may possess alleles for high glucosinolates which are only expressed in particular genetic backgrounds. Gsl-elong and Gsl-alk, loci which regulate the ratio of individual aliphatic glucosinolates, were also mapped. Gsl-elong-1 and Gsl-elong-2, which control elongation of the α-amino-acid precursors, mapped to LG 18 and LG 20 and were coincident with GSL loci which regulate total seed aliphatic glucosinolates. A third tentative QTL, which regulates side-chain elongation, was tentatively mapped to LG 12. Gsl-alk, which regulates H3CS-removal and side-chain de-saturation, mapped to LG 20.
Similar content being viewed by others
References
Andersen AS, Muir RM (1966) Auxin activity of glucobrassicin. Physiol Plant 19:1038–1048
Benn M (1977) Glucosinolates. Pure Appl Chem 49:197–210
Duncan AJ (1991) Glucosinolates: In: D'Mello JPF, Duffus CM, Duffus JH (eds) Toxic substances in crop plants. The Royal Society of Chemistry, pp 127–147
Edwards MD (1992) Use of molecular markers in the evaluation and introgression of genetic diversity for quantitative traits. Fields Crop Res 29:241–260
Fenwick GR (1984) Rapeseed as an animal feeding stuff — the problems and analysis of glucosinolates. J Assoc Publ Anal 22: 117–130
Fenwick GR, Heaney RK, Mullin WJ (1983) Glucosinolates and their breakdown products in food and food plants. CRC Crit Rev Food Sci Nutr 18:123–201
Ferreira ME, Williams PH, Osborn TC (1994) RFLP mapping of Brassica napus using doubled-haploid lines. Theor Appl Genet 89:615–621
Gland A, Röbbelen G, Thies W (1981) Variation in alkenyl glucosinolates in seeds of Brassica species. Z Pflanzenzüchtg 87: 96–110
Heaney RK, Spinks EA, Hanley AB, Fenwick GR (1986) Analysis of glucosinolates in rapeseed. Technical Bulletin, AFRC Food Research Institute, Norwich, UK
Horáková K (1966) Cytotoxicity of natural and synthetic isothiocyanates. Naturwissenschaften 15:383–384
Giamoustaris A, Mithen R (1995) The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus spp. oleifera) on its interaction with specialist and generalist pests. Ann Appl Biol 126:347–363
Kondra ZP, Stefansson BR (1970) Inheritance of the major glucosinolates of rapeseed (Brassica napus) meal. Can J Plant Sci 50: 643–647
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Botsein D (1989) Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Landry BS, Hubert N, Etoh T, Harada JJ, Lincoln SE (1991) A genetic map for Brassica napus based on restriction fragment length polymorphisms detected with expressed DNA sequences. Genome 34:543–55
Larsen PO (1981) Glucosinolates. In: Stumpf PK, Conn EE (eds) The Biochemistry of Plants, vol 7. Academic Press, London, pp 502–523
Larsen LM, Nielsen JK, Sørensen H (1992) Host-plant recognition in monophagous weevils: specialization of Ceutorhynchus inaffectatus to glucosinolates from its host plant Hesperis matronalis. Entomol Exp Appl 64:49–55
Lincoln SE, Lander ES (1990) Mapping genes for quantitative traits using MAPMAKER/QTL. Whitehead Institute for Biomedical esearch Technical Report, Cambridge, Massachusetts
Magrath R, Herron C, Giamoustaris A, Mithen R (1993) The inheritance of aliphatic glucosinolates in Brassica napus. Plant Breed 111:55–72
Magrath R, Bano F, Morgner M, Parkin I, Sharpe A, Lister C, Dean C, Turner J, Lydiate D, Mithen R (1994) Genetics of aliphatic glucosinolates. I. Side-chain elongation in Brassica napus and Arabidopsis thaliana. Heredity 72:290–299
Mithen R (1992) Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 63:71–83
Mithen R, Clarke J, Lister C, Dean C (1995) Genetics of aliphatic glucosinolates. III. Side-chain structure of aliphatic glucosinolates in Arabidopsis thaliana. Heredity 74:210–215.
Paterson AH, Lander ES, Hewitt JD, Peterson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335:721–726
Parkin I, Magrath R, Keith D, Sharpe A, Mithen R, Lydiate D (1994). Genetics of aliphatic glucosinolates. II. Hydroxylation of alkenyl glucosinolates in Brassica napus. Heredity 72:594–598
Röbbelen G, Thies W (1980) Variation in rapeseed glucosinolates and breeding for improved meal quality. In: Tsunoda S, Hinata K, Gómez Campo. (eds) Brassica crops and wild allies. Biology and breeding. Japan Scientific Societies Press, Tokyo, pp 285–299
Rücker B, Rudlof, E (1991) Investigations on the inheritance of the glucosinolate content in seeds of winter oilseed rape (Brassica napus L.) Proc 8th Int Rapeseed Congr, Saskatoon, Canada, pp 191–196
Thormann CE, Ferreira ME, Camargo LEA, Tivang JG, Osborn TC (1994) Comparison of RFLP and RAPD markers for estimating genetic relationships within and between crucifer species. Theor Appl Genet 88:973–980
Weller JI (1986) Maximum-likelihood techniques for the mapping and analysis of quantitative trait loci with the aid of genetic markers. Biometrics 42:627–640
Author information
Authors and Affiliations
Additional information
Communicated by G. E. Hart
Rights and permissions
About this article
Cite this article
Toroser, D., Thormann, C.E., Osborn, T.C. et al. RFLP mapping of quantitative trait loci controlling seed aliphatic-glucosinolate content in oilseed rape (Brassica napus L). Theoret. Appl. Genetics 91, 802–808 (1995). https://doi.org/10.1007/BF00220963
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220963