Abstract
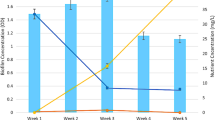
[3H]leucine incorporation into protein, as a method of measuring bacterial biomass production (BBP), was adapted to epiphytic bacteria. Incorporation of the isotope was saturated at concentrations higher than 400 nM. Disruption of thicker biofilms by sonication resulted in higher values of BBP and ratios of BBP/biomass when compared to those of intact biofilm. Thin biofilms formed early in the decomposition process did not show this phenomenon. These results support to evidence that more internally located cells of the matrices either have greatly reduced access to the leucine from the overlying medium or that fast recycling of leucine occurs in the biofilm.
Similar content being viewed by others
References
Benner R, Hodson RE, Kirchman D (1988) Bacterial abundance and production on mangrove leaves during initial stages of leaching and biodegradation. Arch Hydrobiol Beih Ergebnisse Limnol 31:19–26
Blum LK, Mills AL (1991) Microbial growth and activity during the initial stages of seagrass decomposition. Mar Ecol Prog Ser 70:73–82
Burkholder JM, Wetzel RG, Klomparens KL (1990) Direct comparison of phosphate uptake by adnate and loosely attached microalgae within an intact biofilm matrix. Appl Environ Microbiol 56:2882–2890
Caldwell DE, Corber DR, Lawrence JR (1992) Confocal laser microscopy and digital image analysis in microbial ecology. Adv Microbial Ecol 12:1–67
Chin-Leo G, Benner R (1991) Dynamics of bacterioplankton abundance and production in seagrass communities of a hypesaline lagoon. Mar Ecol Prog Ser 73:219–230
Coveney MF, Wetzel RG (1988) Experimental evaluation of conversion factors for the [3H]thymidine incorporation assay of bacterial secondary productivity. Appl Environ Microbiol 54:2018–2026
Fallon RD, Newell SY (1986) Thymidine incorporation by the microbial community of standing dead Spartina alterniflora. Appl Environ Microbiol 52;1206–1208
Findlay S (1993) Thymidine incorporation into DNA as an estimate of sediment bacterial production. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Aquatic microbial ecology. Lewis Publ., Boca Raton, Florida, pp 505–508
Findlay SK, Arsuffi TL (1989) Microbial growth and detritus transformations during decomposition of leaf litter in a stream. Freshwater Biol 21:261–269
Findlay SK, Meyer JL, Risley R (1986) Benthic bacterial biomass and production in two blackwater rivers. Can J Fish Aquat Sci 43:1271–1276
Fry JF (1990) Direct methods and biomass estimation. Methods Microbiol 22:41–81
Jørgensen NOG (1992) Incorporation of [3H]leucine and [3H]valine into protein of freshwater bacteria: uptake kinetics and intracellular isotope dilution. Appl Environ Microbiol 58:3638–3646
Kenworthy WJ, Currin CA, Fonseca MS, Smith G (1989) Production, decomposition, and heterotrophic utilization of the seagrass Halophila decipiens in a submarine canyon. Mar Ecol Prog Ser 51:277–290
Kirchman DL (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Aquatic microbial ecology. Lewis Publ., Boca Raton, Florida, pp 509–512
Kirchman DL, Ducklow HW (1993) Estimating conversion factors for the thymidine and leucine methods for measuring bacterial production. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Aquatic microbial ecology. Lewis Publ., Boca Raton, Florida, pp 513–517
Kirchman DL, Hoch MP (1988) Bacterial production in the Delaware bay estuary estimated from thymidine and leucine incorporation rates. Mar Ecol Prog Ser 45:169–178
Kirchman DL, Nees EK, Hodson R (1985) Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49:599–607
Moran MA, Hodson RE (1989) Bacterial secondary production on vascular plant detritus relationships to detritus composition and degradation rate. Appl Environ Microbiol 55:2178–2189
Moriarty DJW (1990) Techniques for estimating bacterial growth rates and production of biomass in aquatic environments. Methods Microbiol 22:211–234
Porter K, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Velji MI, Albright LJ (1986) Microscopic enumeration of attached marine bacteria of seawater, marine sediment, fecal matter, and kelp blade samples following pyrophosphate and ultrasound treatments. Can J Microbiol 32:121–126
Wetzel RG (1993) Microcommunities and microgradients:linking nutrient regeneration, microbial mutualism, and high sustained aquatic primary production. Netherlands J Aquat Ecol 27:1–7
Wetzel RG, Likens GE (1991) Limnological analyses. Springer-Verlag, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomaz, S.M., Wetzel, R.G. [3H]Leucine incorporation methodology to estimate epiphytic bacterial biomass production. Microb Ecol 29, 63–70 (1995). https://doi.org/10.1007/BF00217423
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00217423